CD16 Antibody - #DF7007
| Product: | CD16 Antibody |
| Catalog: | DF7007 |
| Description: | Rabbit polyclonal antibody to CD16 |
| Application: | WB IHC |
| Cited expt.: | WB, IHC |
| Reactivity: | Human, Mouse, Rat |
| Mol.Wt.: | 29kD, 50kD; 29kD(Calculated). |
| Uniprot: | P08637 |
| RRID: | AB_2838963 |
Related Downloads
Protocols
Product Info
*The optimal dilutions should be determined by the end user. For optimal experimental results, antibody reuse is not recommended.
*Tips:
WB: For western blot detection of denatured protein samples. IHC: For immunohistochemical detection of paraffin sections (IHC-p) or frozen sections (IHC-f) of tissue samples. IF/ICC: For immunofluorescence detection of cell samples. ELISA(peptide): For ELISA detection of antigenic peptide.
Cite Format: Affinity Biosciences Cat# DF7007, RRID:AB_2838963.
Fold/Unfold
CD 16; CD 16a; CD16; CD16a; CD16a antigen; CD16B; CD16b antigen; Fc fragment of IgG; Fc fragment of IgG low affinity IIIa receptor (CD16); Fc fragment of IgG low affinity IIIa receptor; Fc fragment of IgG receptor IIIa; Fc fragment of IgG, low affinity III, receptor (CD16); Fc fragment of IgG, low affinity III, receptor for (CD16); Fc fragment of IgG, low affinity IIIa, receptor (CD16); Fc fragment of IgG, low affinity IIIa, receptor (CD16a); Fc fragment of IgG, low affinity IIIa, receptor for; Fc fragment of IgG, low affinity IIIb, receptor (CD16b); Fc fragment of IgG, low affinity IIIb, receptor for (CD16); Fc gamma R3; Fc gamma receptor III 2 (CD 16); Fc gamma receptor III A; Fc gamma receptor IIIA; Fc gamma receptor IIIb (CD 16); Fc gamma RIII alpha; Fc gamma RIII; Fc gamma RIII beta; Fc gamma RIIIa; Fc gamma RIIIb; Fc of IgG; Fc-gamma receptor III2 (CD 16); Fc-gamma receptor III2 (CD16); Fc-gamma receptor IIIb (CD16); Fc-gamma RIII; Fc-gamma RIII-alpha; Fc-gamma RIIIa; FCG 3; FCG3; FCG3A_HUMAN; FCgammaRIIIA; FCGR 3; FCGR 3A; FCGR3; FCGR3A; FCGR3A protein; FCGRIII; FCGRIII-2; FcR 10; FcR-10; FcR10; FcRIII; FcRIIIa; IGFR 3; IGFR3; IgG Fc receptor III 1; IgG Fc receptor III 2; IgG Fc receptor III-2; IMD20; immunoglobulin G Fc receptor III; immunoglobulin G Fc receptor III-2; Low affinity IIIa receptor; Low affinity immunoglobulin gamma Fc region receptor III A; Low affinity immunoglobulin gamma Fc region receptor III-A; Low affinity immunoglobulin gamma Fc region receptor IIIB; neutrophil-specific antigen NA;
Immunogens
A synthesized peptide derived from human CD16, corresponding to a region within C-terminal amino acids.
Expressed on natural killer cells, macrophages, subpopulation of T-cells, immature thymocytes and placental trophoblasts.
- P08637 FCG3A_HUMAN:
- Protein BLAST With
- NCBI/
- ExPASy/
- Uniprot
MWQLLLPTALLLLVSAGMRTEDLPKAVVFLEPQWYRVLEKDSVTLKCQGAYSPEDNSTQWFHNESLISSQASSYFIDAATVDDSGEYRCQTNLSTLSDPVQLEVHIGWLLLQAPRWVFKEEDPIHLRCHSWKNTALHKVTYLQNGKGRKYFHHNSDFYIPKATLKDSGSYFCRGLFGSKNVSSETVNITITQGLAVSTISSFFPPGYQVSFCLVMVLLFAVDTGLYFSVKTNIRSSTRDWKDHKFKWRKDPQDK
Research Backgrounds
Receptor for the Fc region of IgG. Binds complexed or aggregated IgG and also monomeric IgG. Mediates antibody-dependent cellular cytotoxicity (ADCC) and other antibody-dependent responses, such as phagocytosis.
Glycosylated. Contains high mannose- and complex-type oligosaccharides. Glycosylation at Asn-180 is mandatory for high affinity binding to the Fc and for discrimination between fucosylated and afucosylated IgG glycoforms.
The soluble form is produced by a proteolytic cleavage.
Cell membrane>Single-pass type I membrane protein. Secreted.
Note: Exists also as a soluble receptor.
Expressed on natural killer cells, macrophages, subpopulation of T-cells, immature thymocytes and placental trophoblasts.
Research Fields
· Cellular Processes > Transport and catabolism > Phagosome. (View pathway)
· Human Diseases > Infectious diseases: Parasitic > Leishmaniasis.
· Human Diseases > Infectious diseases: Bacterial > Staphylococcus aureus infection.
· Human Diseases > Infectious diseases: Bacterial > Tuberculosis.
· Human Diseases > Immune diseases > Systemic lupus erythematosus.
· Organismal Systems > Development > Osteoclast differentiation. (View pathway)
· Organismal Systems > Immune system > Natural killer cell mediated cytotoxicity. (View pathway)
· Organismal Systems > Immune system > Fc gamma R-mediated phagocytosis. (View pathway)
References
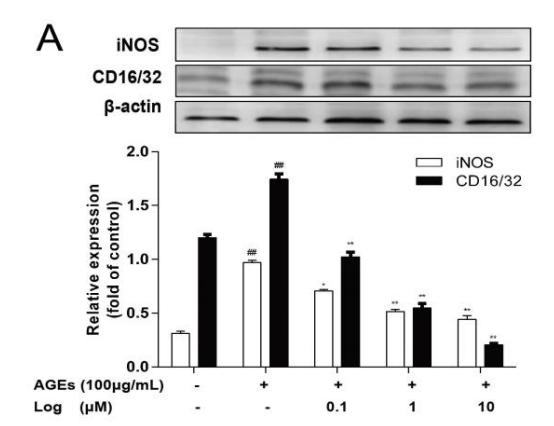
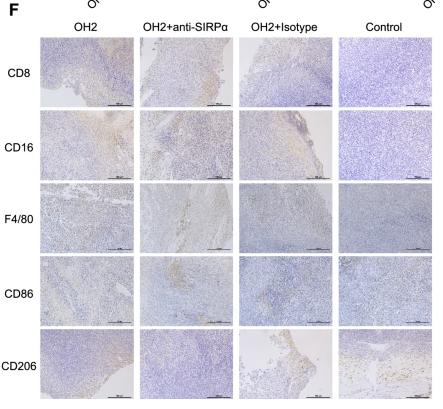
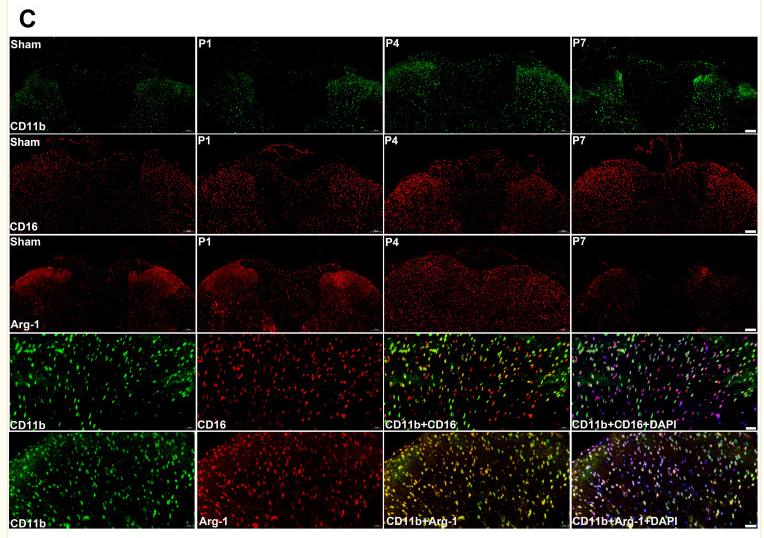
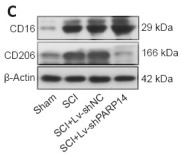
Application: IHC Species: Mouse Sample:
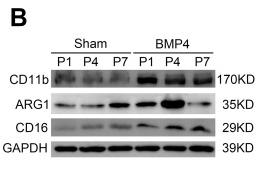
Application: WB Species: Mouse Sample:
Application: WB Species: mice Sample: RAW264.7 cells
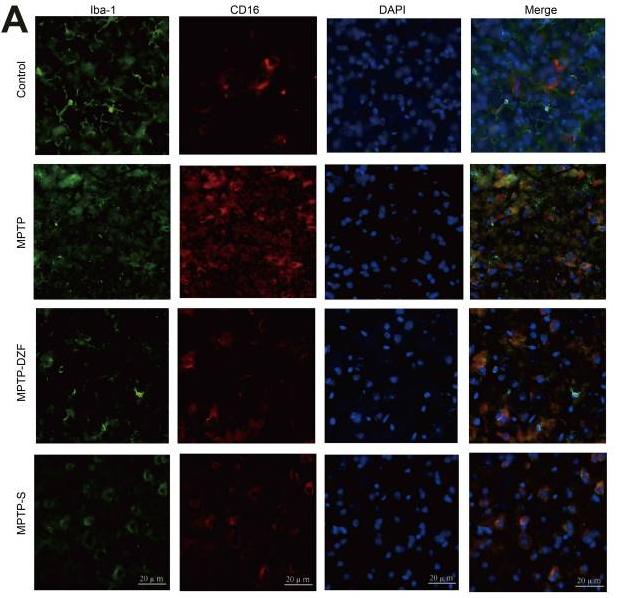
Application: IF/ICC Species: Mice Sample: midbrain tissue
Application: WB Species: Rat Sample: spinal cord
Application: IF/ICC Species: Rat Sample: spinal cord
Restrictive clause
Affinity Biosciences tests all products strictly. Citations are provided as a resource for additional applications that have not been validated by Affinity Biosciences. Please choose the appropriate format for each application and consult Materials and Methods sections for additional details about the use of any product in these publications.
For Research Use Only.
Not for use in diagnostic or therapeutic procedures. Not for resale. Not for distribution without written consent. Affinity Biosciences will not be held responsible for patent infringement or other violations that may occur with the use of our products. Affinity Biosciences, Affinity Biosciences Logo and all other trademarks are the property of Affinity Biosciences LTD.