ATF6 Antibody - #DF6009
| Product: | ATF6 Antibody |
| Catalog: | DF6009 |
| Description: | Rabbit polyclonal antibody to ATF6 |
| Application: | WB IHC IF/ICC |
| Cited expt.: | WB, IHC, IF/ICC |
| Reactivity: | Human, Mouse, Rat |
| Prediction: | Pig, Zebrafish, Horse, Sheep, Rabbit, Dog, Xenopus |
| Mol.Wt.: | 50~75kD(cleaved),90~100kD(full); 75kD(Calculated). |
| Uniprot: | P18850 |
| RRID: | AB_2833019 |
Product Info
*The optimal dilutions should be determined by the end user. For optimal experimental results, antibody reuse is not recommended.
*Tips:
WB: For western blot detection of denatured protein samples. IHC: For immunohistochemical detection of paraffin sections (IHC-p) or frozen sections (IHC-f) of tissue samples. IF/ICC: For immunofluorescence detection of cell samples. ELISA(peptide): For ELISA detection of antigenic peptide.
Cite Format: Affinity Biosciences Cat# DF6009, RRID:AB_2833019.
Fold/Unfold
Activating transcription factor 6 alpha; Activating transcription factor 6; ATF 6; ATF6 alpha; ATF6; ATF6-alpha; ATF6A; ATF6A_HUMAN; cAMP dependent transcription factor ATF 6 alpha; cAMP-dependent transcription factor ATF-6 alpha; Cyclic AMP dependent transcription factor ATF 6 alpha; DKFZp686P2194; ESTM49; FLJ21663; Processed cyclic AMP dependent transcription factor ATF 6 alpha; Processed cyclic AMP-dependent transcription factor ATF-6 alpha;
Immunogens
A synthesized peptide derived from human ATF6, corresponding to a region within the internal amino acids.
- P18850 ATF6A_HUMAN:
- Protein BLAST With
- NCBI/
- ExPASy/
- Uniprot
MGEPAGVAGTMESPFSPGLFHRLDEDWDSALFAELGYFTDTDELQLEAANETYENNFDNLDFDLDLMPWESDIWDINNQICTVKDIKAEPQPLSPASSSYSVSSPRSVDSYSSTQHVPEELDLSSSSQMSPLSLYGENSNSLSSAEPLKEDKPVTGPRNKTENGLTPKKKIQVNSKPSIQPKPLLLPAAPKTQTNSSVPAKTIIIQTVPTLMPLAKQQPIISLQPAPTKGQTVLLSQPTVVQLQAPGVLPSAQPVLAVAGGVTQLPNHVVNVVPAPSANSPVNGKLSVTKPVLQSTMRNVGSDIAVLRRQQRMIKNRESACQSRKKKKEYMLGLEARLKAALSENEQLKKENGTLKRQLDEVVSENQRLKVPSPKRRVVCVMIVLAFIILNYGPMSMLEQDSRRMNPSVSPANQRRHLLGFSAKEAQDTSDGIIQKNSYRYDHSVSNDKALMVLTEEPLLYIPPPPCQPLINTTESLRLNHELRGWVHRHEVERTKSRRMTNNQQKTRILQGALEQGSNSQLMAVQYTETTSSISRNSGSELQVYYASPRSYQDFFEAIRRRGDTFYVVSFRRDHLLLPATTHNKTTRPKMSIVLPAININENVINGQDYEVMMQIDCQVMDTRILHIKSSSVPPYLRDQQRNQTNTFFGSPPAATEATHVVSTIPESLQ
Predictions
Score>80(red) has high confidence and is suggested to be used for WB detection. *The prediction model is mainly based on the alignment of immunogen sequences, the results are for reference only, not as the basis of quality assurance.
High(score>80) Medium(80>score>50) Low(score<50) No confidence
Research Backgrounds
Transmembrane glycoprotein of the endoplasmic reticulum that functions as a transcription activator and initiates the unfolded protein response (UPR) during endoplasmic reticulum stress. Cleaved upon ER stress, the N-terminal processed cyclic AMP-dependent transcription factor ATF-6 alpha translocates to the nucleus where it activates transcription of genes involved in the UPR. Binds DNA on the 5'-CCAC[GA]-3'half of the ER stress response element (ERSE) (5'-CCAAT-N(9)-CCAC[GA]-3') and of ERSE II (5'-ATTGG-N-CCACG-3'). Binding to ERSE requires binding of NF-Y to ERSE. Could also be involved in activation of transcription by the serum response factor. May play a role in foveal development and cone function in the retina.
During unfolded protein response, a fragment of approximately 50 kDa containing the cytoplasmic transcription factor domain is released by proteolysis. The cleavage seems to be performed sequentially by site-1 and site-2 proteases.
N-glycosylated. The glycosylation status may serve as a sensor for ER homeostasis, resulting in ATF6 activation to trigger the unfolded protein response (UPR).
Phosphorylated in vitro by MAPK14/P38MAPK.
Endoplasmic reticulum membrane>Single-pass type II membrane protein.
Nucleus.
Note: Under ER stress the cleaved N-terminal cytoplasmic domain translocates into the nucleus. THBS4 promotes its nuclear shuttling.
Ubiquitous.
The basic domain functions as a nuclear localization signal.
The basic leucine-zipper domain is sufficient for association with the NF-Y trimer and binding to ERSE.
Belongs to the bZIP family. ATF subfamily.
Research Fields
· Genetic Information Processing > Folding, sorting and degradation > Protein processing in endoplasmic reticulum. (View pathway)
· Human Diseases > Neurodegenerative diseases > Alzheimer's disease.
References
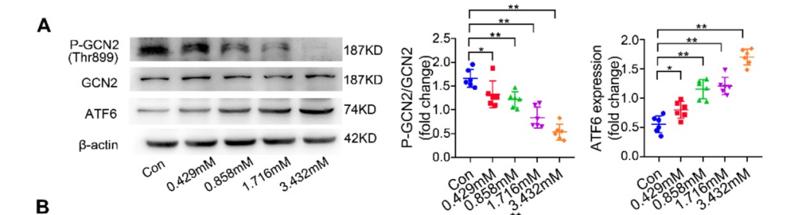
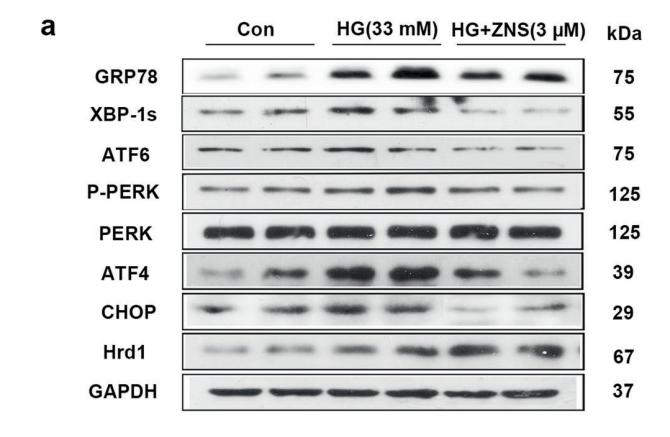
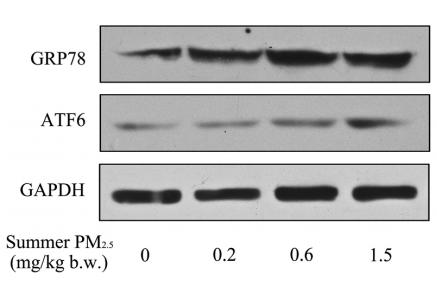
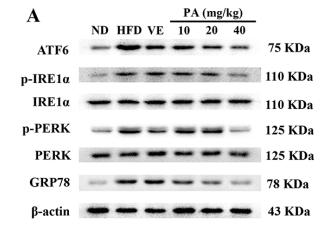
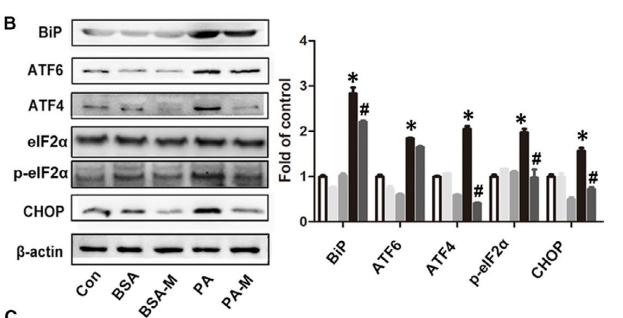
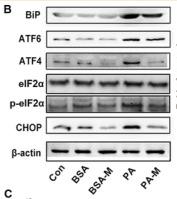
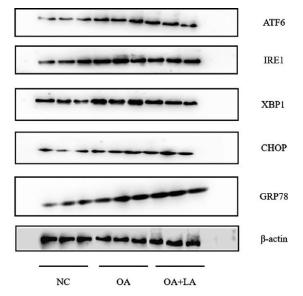
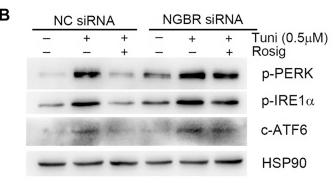
Application: WB Species: mouse Sample: Heart
Application: WB Species: mice Sample: NRCMs
Application: WB Species: Human Sample: Hep-G2 cells
Application: WB Species: Human Sample:
Application: WB Species: Human Sample: HBE135-E6E7 cells
Application: WB Species: Human Sample: HUVEC cells
Application: WB Species: rat Sample: liver
Restrictive clause
Affinity Biosciences tests all products strictly. Citations are provided as a resource for additional applications that have not been validated by Affinity Biosciences. Please choose the appropriate format for each application and consult Materials and Methods sections for additional details about the use of any product in these publications.
For Research Use Only.
Not for use in diagnostic or therapeutic procedures. Not for resale. Not for distribution without written consent. Affinity Biosciences will not be held responsible for patent infringement or other violations that may occur with the use of our products. Affinity Biosciences, Affinity Biosciences Logo and all other trademarks are the property of Affinity Biosciences LTD.