TRAF3 Antibody - #AF5380
| Product: | TRAF3 Antibody |
| Catalog: | AF5380 |
| Description: | Rabbit polyclonal antibody to TRAF3 |
| Application: | WB IF/ICC |
| Cited expt.: | WB, IF/ICC |
| Reactivity: | Human, Mouse, Rat |
| Prediction: | Pig, Bovine, Horse, Sheep, Rabbit, Dog, Chicken, Xenopus |
| Mol.Wt.: | 68 kDa, 55 kDa; 64kD(Calculated). |
| Uniprot: | Q13114 |
| RRID: | AB_2837865 |
Product Info
*The optimal dilutions should be determined by the end user. For optimal experimental results, antibody reuse is not recommended.
*Tips:
WB: For western blot detection of denatured protein samples. IHC: For immunohistochemical detection of paraffin sections (IHC-p) or frozen sections (IHC-f) of tissue samples. IF/ICC: For immunofluorescence detection of cell samples. ELISA(peptide): For ELISA detection of antigenic peptide.
Cite Format: Affinity Biosciences Cat# AF5380, RRID:AB_2837865.
Fold/Unfold
CAP 1; CAP-1; CAP1; CD40 associated protein 1; CD40 binding protein; CD40 bp; CD40 receptor associated factor 1; CD40 receptor-associated factor 1; CD40-binding protein; CD40BP; CRAF 1; CRAF1; IIAE5; LAP 1; LAP1; LMP1 associated protein; LMP1-associated protein 1; MIPT3; TNF receptor associated factor 3; TNF receptor-associated factor 3; TRAF 3; Traf3; TRAF3_HUMAN;
Immunogens
A synthesized peptide derived from human TRAF3, corresponding to a region within the internal amino acids.
- Q13114 TRAF3_HUMAN:
- Protein BLAST With
- NCBI/
- ExPASy/
- Uniprot
MESSKKMDSPGALQTNPPLKLHTDRSAGTPVFVPEQGGYKEKFVKTVEDKYKCEKCHLVLCSPKQTECGHRFCESCMAALLSSSSPKCTACQESIVKDKVFKDNCCKREILALQIYCRNESRGCAEQLMLGHLLVHLKNDCHFEELPCVRPDCKEKVLRKDLRDHVEKACKYREATCSHCKSQVPMIALQKHEDTDCPCVVVSCPHKCSVQTLLRSELSAHLSECVNAPSTCSFKRYGCVFQGTNQQIKAHEASSAVQHVNLLKEWSNSLEKKVSLLQNESVEKNKSIQSLHNQICSFEIEIERQKEMLRNNESKILHLQRVIDSQAEKLKELDKEIRPFRQNWEEADSMKSSVESLQNRVTELESVDKSAGQVARNTGLLESQLSRHDQMLSVHDIRLADMDLRFQVLETASYNGVLIWKIRDYKRRKQEAVMGKTLSLYSQPFYTGYFGYKMCARVYLNGDGMGKGTHLSLFFVIMRGEYDALLPWPFKQKVTLMLMDQGSSRRHLGDAFKPDPNSSSFKKPTGEMNIASGCPVFVAQTVLENGTYIKDDTIFIKVIVDTSDLPDP
Predictions
Score>80(red) has high confidence and is suggested to be used for WB detection. *The prediction model is mainly based on the alignment of immunogen sequences, the results are for reference only, not as the basis of quality assurance.
High(score>80) Medium(80>score>50) Low(score<50) No confidence
Research Backgrounds
Regulates pathways leading to the activation of NF-kappa-B and MAP kinases, and plays a central role in the regulation of B-cell survival. Part of signaling pathways leading to the production of cytokines and interferon. Required for normal antibody isotype switching from IgM to IgG. Plays a role T-cell dependent immune responses. Plays a role in the regulation of antiviral responses. Is an essential constituent of several E3 ubiquitin-protein ligase complexes. May have E3 ubiquitin-protein ligase activity and promote 'Lys-63'-linked ubiquitination of target proteins. Inhibits activation of NF-kappa-B in response to LTBR stimulation. Inhibits TRAF2-mediated activation of NF-kappa-B. Down-regulates proteolytic processing of NFKB2, and thereby inhibits non-canonical activation of NF-kappa-B. Promotes ubiquitination and proteasomal degradation of MAP3K14.
Undergoes 'Lys-48'-linked polyubiquitination, leading to its proteasomal degradation in response to signaling by TNFSF13B, TLR4 or through CD40. 'Lys-48'-linked polyubiquitinated form is deubiquitinated by OTUD7B, preventing TRAF3 proteolysis and over-activation of non-canonical NF-kappa-B. Undergoes 'Lys-63'-linked ubiquitination during early stages of virus infection, and 'Lys-48'-linked ubiquitination during later stages. Undergoes both 'Lys-48'-linked and 'Lys-63'-linked ubiquitination in response to TLR3 and TLR4 signaling. Deubiquitinated by OTUB1, OTUB2 and OTUD5.
Cytoplasm. Endosome. Mitochondrion.
Note: Undergoes endocytosis together with TLR4 upon LPS signaling (By similarity). Associated with mitochondria in response to virus.
The MATH/TRAF domain binds to receptor cytoplasmic domains.
The Ring-type zinc finger domain is required for its function in down-regulation of NFKB2 proteolytic processing.
Belongs to the TNF receptor-associated factor family. A subfamily.
Research Fields
· Environmental Information Processing > Signal transduction > NF-kappa B signaling pathway. (View pathway)
· Environmental Information Processing > Signal transduction > TNF signaling pathway. (View pathway)
· Human Diseases > Infectious diseases: Viral > Hepatitis C.
· Human Diseases > Infectious diseases: Viral > Human papillomavirus infection.
· Human Diseases > Infectious diseases: Viral > Herpes simplex infection.
· Human Diseases > Infectious diseases: Viral > Epstein-Barr virus infection.
· Human Diseases > Cancers: Overview > Pathways in cancer. (View pathway)
· Human Diseases > Cancers: Overview > Viral carcinogenesis.
· Human Diseases > Cancers: Specific types > Small cell lung cancer. (View pathway)
· Organismal Systems > Immune system > Toll-like receptor signaling pathway. (View pathway)
· Organismal Systems > Immune system > NOD-like receptor signaling pathway. (View pathway)
· Organismal Systems > Immune system > RIG-I-like receptor signaling pathway. (View pathway)
· Organismal Systems > Immune system > IL-17 signaling pathway. (View pathway)
References
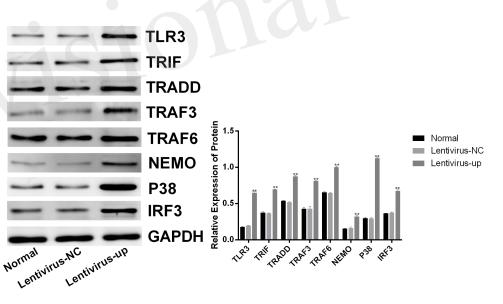
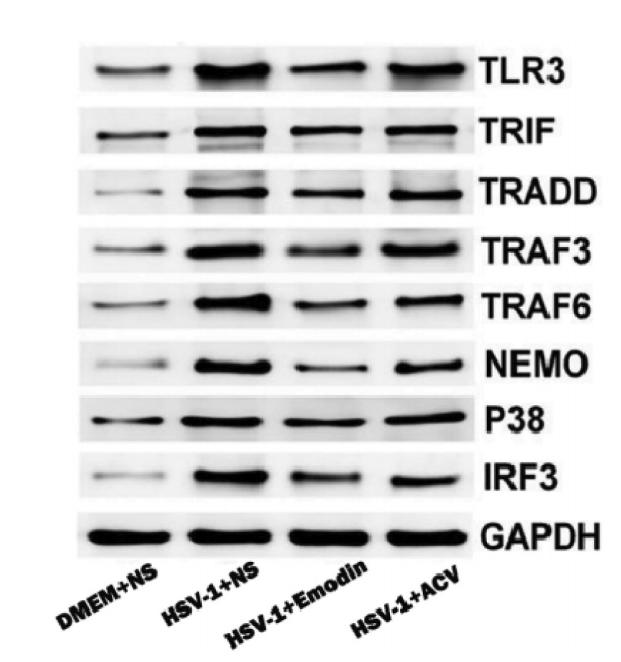
Application: WB Species: human Sample: HepG2.2.15 cells
Application: IF/ICC Species: human Sample: HepG2.2.15 cells
Application: WB Species: Human Sample: HepG2.2.15 cells
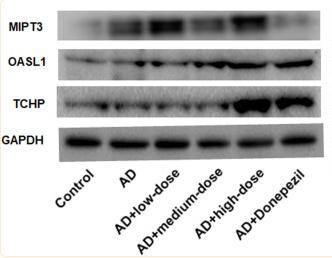
Application: WB Species: mouse Sample: BV-2 cells
Application: WB Species: Mouse Sample:
Application: WB Species: Mice Sample: brain tissues
Restrictive clause
Affinity Biosciences tests all products strictly. Citations are provided as a resource for additional applications that have not been validated by Affinity Biosciences. Please choose the appropriate format for each application and consult Materials and Methods sections for additional details about the use of any product in these publications.
For Research Use Only.
Not for use in diagnostic or therapeutic procedures. Not for resale. Not for distribution without written consent. Affinity Biosciences will not be held responsible for patent infringement or other violations that may occur with the use of our products. Affinity Biosciences, Affinity Biosciences Logo and all other trademarks are the property of Affinity Biosciences LTD.