PPAR alpha Antibody - #AF5301
| Product: | PPAR alpha Antibody |
| Catalog: | AF5301 |
| Description: | Rabbit polyclonal antibody to PPAR alpha |
| Application: | WB IHC IF/ICC |
| Cited expt.: | WB, IHC, IF/ICC |
| Reactivity: | Human, Mouse, Rat |
| Prediction: | Bovine, Horse, Sheep, Rabbit, Dog, Xenopus |
| Mol.Wt.: | 52 kDa; 52kD(Calculated). |
| Uniprot: | Q07869 |
| RRID: | AB_2837786 |
Related Downloads
Protocols
Product Info
*The optimal dilutions should be determined by the end user. For optimal experimental results, antibody reuse is not recommended.
*Tips:
WB: For western blot detection of denatured protein samples. IHC: For immunohistochemical detection of paraffin sections (IHC-p) or frozen sections (IHC-f) of tissue samples. IF/ICC: For immunofluorescence detection of cell samples. ELISA(peptide): For ELISA detection of antigenic peptide.
Cite Format: Affinity Biosciences Cat# AF5301, RRID:AB_2837786.
Fold/Unfold
hPPAR; MGC2237; MGC2452; NR1C1; Nuclear receptor subfamily 1 group C member 1; OTTHUMP00000197740; OTTHUMP00000197741; Peroxisome proliferative activated receptor alpha; Peroxisome proliferator activated receptor alpha; Peroxisome proliferator-activated receptor alpha; PPAR; PPAR-alpha; ppara; PPARA_HUMAN; PPARalpha;
Immunogens
A synthesized peptide derived from human PPAR alpha, corresponding to a region within N-terminal amino acids.
Skeletal muscle, liver, heart and kidney. Expressed in monocytes (PubMed:28167758).
- Q07869 PPARA_HUMAN:
- Protein BLAST With
- NCBI/
- ExPASy/
- Uniprot
MVDTESPLCPLSPLEAGDLESPLSEEFLQEMGNIQEISQSIGEDSSGSFGFTEYQYLGSCPGSDGSVITDTLSPASSPSSVTYPVVPGSVDESPSGALNIECRICGDKASGYHYGVHACEGCKGFFRRTIRLKLVYDKCDRSCKIQKKNRNKCQYCRFHKCLSVGMSHNAIRFGRMPRSEKAKLKAEILTCEHDIEDSETADLKSLAKRIYEAYLKNFNMNKVKARVILSGKASNNPPFVIHDMETLCMAEKTLVAKLVANGIQNKEAEVRIFHCCQCTSVETVTELTEFAKAIPGFANLDLNDQVTLLKYGVYEAIFAMLSSVMNKDGMLVAYGNGFITREFLKSLRKPFCDIMEPKFDFAMKFNALELDDSDISLFVAAIICCGDRPGLLNVGHIEKMQEGIVHVLRLHLQSNHPDDIFLFPKLLQKMADLRQLVTEHAQLVQIIKKTESDAALHPLLQEIYRDMY
Predictions
Score>80(red) has high confidence and is suggested to be used for WB detection. *The prediction model is mainly based on the alignment of immunogen sequences, the results are for reference only, not as the basis of quality assurance.
High(score>80) Medium(80>score>50) Low(score<50) No confidence
Research Backgrounds
Ligand-activated transcription factor. Key regulator of lipid metabolism. Activated by the endogenous ligand 1-palmitoyl-2-oleoyl-sn-glycerol-3-phosphocholine (16:0/18:1-GPC). Activated by oleylethanolamide, a naturally occurring lipid that regulates satiety. Receptor for peroxisome proliferators such as hypolipidemic drugs and fatty acids. Regulates the peroxisomal beta-oxidation pathway of fatty acids. Functions as transcription activator for the ACOX1 and P450 genes. Transactivation activity requires heterodimerization with RXRA and is antagonized by NR2C2. May be required for the propagation of clock information to metabolic pathways regulated by PER2.
Nucleus.
Skeletal muscle, liver, heart and kidney. Expressed in monocytes.
Belongs to the nuclear hormone receptor family. NR1 subfamily.
Research Fields
· Environmental Information Processing > Signal transduction > cAMP signaling pathway. (View pathway)
· Human Diseases > Endocrine and metabolic diseases > Insulin resistance.
· Human Diseases > Endocrine and metabolic diseases > Non-alcoholic fatty liver disease (NAFLD).
· Human Diseases > Infectious diseases: Viral > Hepatitis C.
· Organismal Systems > Endocrine system > PPAR signaling pathway.
· Organismal Systems > Endocrine system > Adipocytokine signaling pathway.
· Organismal Systems > Endocrine system > Glucagon signaling pathway.
References
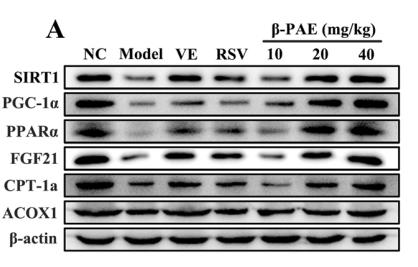
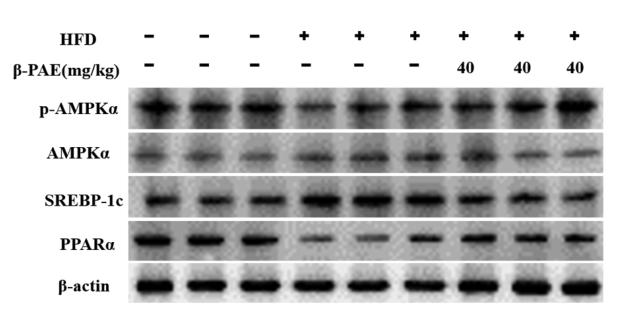
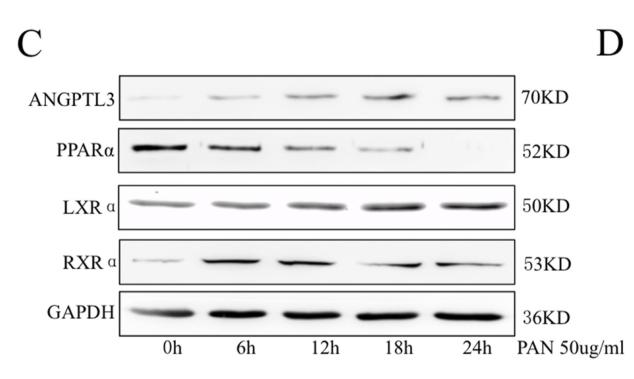
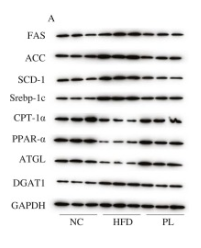
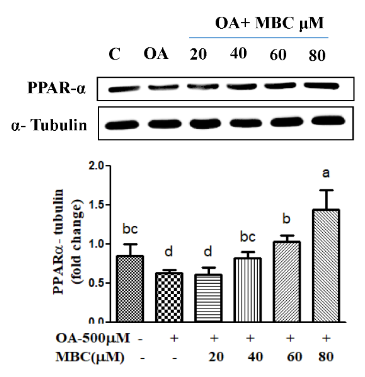
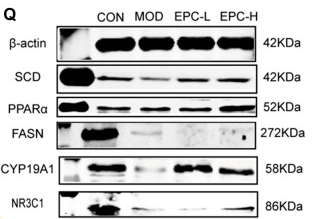
Application: WB Species: Mouse Sample:
Application: WB Species: Mouse Sample: heart
Application: WB Species: Mouse Sample:
Application: WB Species: Mouse Sample:
Application: WB Species: Human Sample: L02 cell
Restrictive clause
Affinity Biosciences tests all products strictly. Citations are provided as a resource for additional applications that have not been validated by Affinity Biosciences. Please choose the appropriate format for each application and consult Materials and Methods sections for additional details about the use of any product in these publications.
For Research Use Only.
Not for use in diagnostic or therapeutic procedures. Not for resale. Not for distribution without written consent. Affinity Biosciences will not be held responsible for patent infringement or other violations that may occur with the use of our products. Affinity Biosciences, Affinity Biosciences Logo and all other trademarks are the property of Affinity Biosciences LTD.