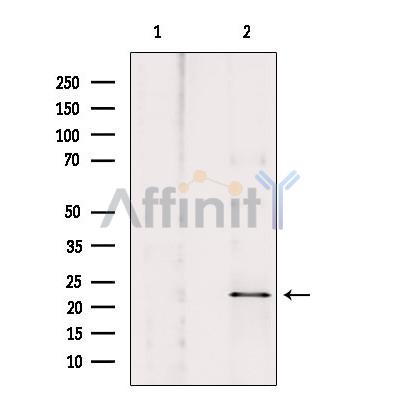
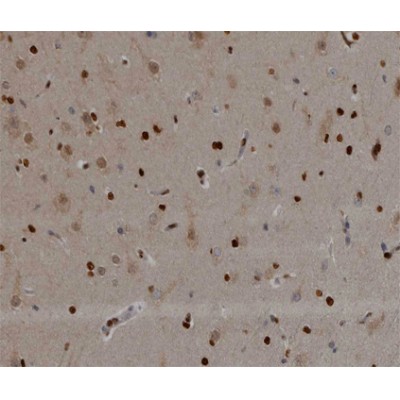
NOL3 Antibody - #AF0118
| Product: | NOL3 Antibody |
| Catalog: | AF0118 |
| Description: | Rabbit polyclonal antibody to NOL3 |
| Application: | WB IHC IF/ICC |
| Reactivity: | Human, Mouse |
| Prediction: | Pig |
| Mol.Wt.: | 23kDa; 23kD(Calculated). |
| Uniprot: | O60936 |
| RRID: | AB_2833302 |
Related Downloads
Protocols
Product Info
*The optimal dilutions should be determined by the end user. For optimal experimental results, antibody reuse is not recommended.
*Tips:
WB: For western blot detection of denatured protein samples. IHC: For immunohistochemical detection of paraffin sections (IHC-p) or frozen sections (IHC-f) of tissue samples. IF/ICC: For immunofluorescence detection of cell samples. ELISA(peptide): For ELISA detection of antigenic peptide.
Cite Format: Affinity Biosciences Cat# AF0118, RRID:AB_2833302.
Fold/Unfold
Apoptosis repressor with CARD; ARC; Muscle enriched cytoplasmic protein; Muscle-enriched cytoplasmic protein; MYC; MYP; Nol3; NOL3_HUMAN; NOP; Nop30; Nucleolar protein 3 (apoptosis repressor with CARD domain); Nucleolar protein 3; Nucleolar protein of 30 kDa;
Immunogens
A synthesized peptide derived from human NOL3, corresponding to a region within C-terminal amino acids.
Highly expressed in heart and skeletal muscle. Detected at low levels in placenta, liver, kidney and pancreas.
- O60936 NOL3_HUMAN:
- Protein BLAST With
- NCBI/
- ExPASy/
- Uniprot
MGNAQERPSETIDRERKRLVETLQADSGLLLDALLARGVLTGPEYEALDALPDAERRVRRLLLLVQGKGEAACQELLRCAQRTAGAPDPAWDWQHVGPGYRDRSYDPPCPGHWTPEAPGSGTTCPGLPRASDPDEAGGPEGSEAVQSGTPEEPEPELEAEASKEAEPEPEPEPELEPEAEAEPEPELEPEPDPEPEPDFEERDESEDS
Predictions
Score>80(red) has high confidence and is suggested to be used for WB detection. *The prediction model is mainly based on the alignment of immunogen sequences, the results are for reference only, not as the basis of quality assurance.
High(score>80) Medium(80>score>50) Low(score<50) No confidence
Research Backgrounds
May be involved in RNA splicing.
Functions as an apoptosis repressor that blocks multiple modes of cell death. Inhibits extrinsic apoptotic pathways through two different ways. Firstly by interacting with FAS and FADD upon FAS activation blocking death-inducing signaling complex (DISC) assembly (By similarity). Secondly by interacting with CASP8 in a mitochondria localization- and phosphorylation-dependent manner, limiting the amount of soluble CASP8 available for DISC-mediated activation (By similarity). Inhibits intrinsic apoptotic pathway in response to a wide range of stresses, through its interaction with BAX resulting in BAX inactivation, preventing mitochondrial dysfunction and release of pro-apoptotic factors. Inhibits calcium-mediated cell death by functioning as a cytosolic calcium buffer, dissociating its interaction with CASP8 and maintaining calcium homeostasis. Negatively regulates oxidative stress-induced apoptosis by phosphorylation-dependent suppression of the mitochondria-mediated intrinsic pathway, by blocking CASP2 activation and BAX translocation (By similarity). Negatively regulates hypoxia-induced apoptosis in part by inhibiting the release of cytochrome c from mitochondria in a caspase-independent manner (By similarity). Also inhibits TNF-induced necrosis by preventing TNF-signaling pathway through TNFRSF1A interaction abrogating the recruitment of RIPK1 to complex I (By similarity). Finally through its role as apoptosis repressor, promotes vascular remodeling through inhibition of apoptosis and stimulation of proliferation, in response to hypoxia (By similarity). Inhibits too myoblast differentiation through caspase inhibition (By similarity).
Phosphorylation at Thr-149 is required for its antiapoptotic effect by blocking death-inducing signaling complex death-inducing signaling complex (DISC) activity through the control of interaction with CASP8. Phosphorylation at Thr-149 results in translocation to mitochondria and this translocation enables the binding to CASP8. Dephosphorylated at Thr-149 by calcineurin; doesn't inhibit the association between FADD and CASP8 and the consequent apoptosis.
Polyubiquitinated by MDM2; promoting proteasomal-dependent degradation in response to apoptotic stimuli.
Nucleus>Nucleolus.
Note: The SR-rich C-terminus mediates nuclear localization.
Cytoplasm.
Cytoplasm. Mitochondrion. Sarcoplasmic reticulum. Membrane>Lipid-anchor.
Note: Phosphorylation at Thr-149 results in translocation to mitochondria. Colocalized with mitochondria in response to oxidative stress.
Highly expressed in heart and skeletal muscle. Detected at low levels in placenta, liver, kidney and pancreas.
CARD is critical for both extrinsic and intrinsic apoptotic pathways (By similarity). CARD domain mediates a protective effect against myocardial ischemia/reperfusion, oxidative stress and TNF-induced necrosis (PubMed:15004034). The calcium binding domain plays a protective role in calcium-mediated cell death (PubMed:15509781).
Restrictive clause
Affinity Biosciences tests all products strictly. Citations are provided as a resource for additional applications that have not been validated by Affinity Biosciences. Please choose the appropriate format for each application and consult Materials and Methods sections for additional details about the use of any product in these publications.
For Research Use Only.
Not for use in diagnostic or therapeutic procedures. Not for resale. Not for distribution without written consent. Affinity Biosciences will not be held responsible for patent infringement or other violations that may occur with the use of our products. Affinity Biosciences, Affinity Biosciences Logo and all other trademarks are the property of Affinity Biosciences LTD.