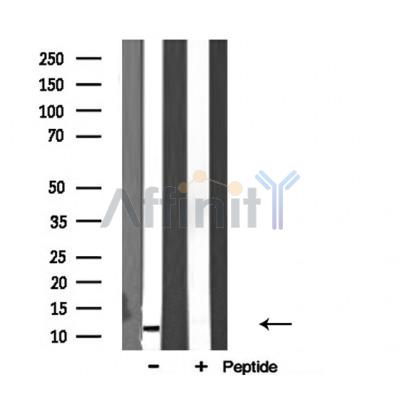
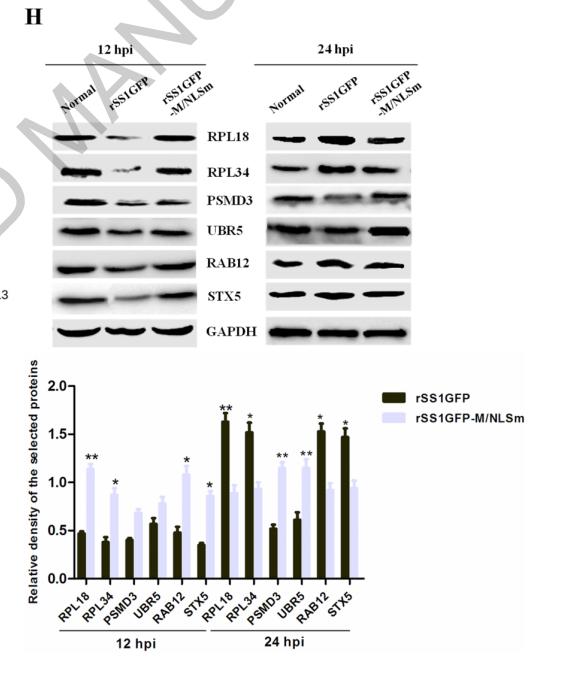
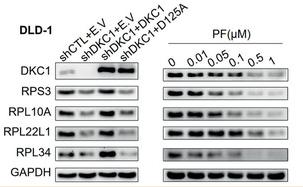
RPL34 Antibody - #DF3708
| Product: | RPL34 Antibody |
| Catalog: | DF3708 |
| Description: | Rabbit polyclonal antibody to RPL34 |
| Application: | WB IHC IF/ICC |
| Cited expt.: | WB |
| Reactivity: | Human, Mouse, Rat, Monkey |
| Prediction: | Pig, Zebrafish, Bovine, Horse, Sheep, Chicken, Xenopus |
| Mol.Wt.: | 13 KD; 13kD(Calculated). |
| Uniprot: | P49207 |
| RRID: | AB_2836072 |
Related Downloads
Protocols
Product Info
*The optimal dilutions should be determined by the end user. For optimal experimental results, antibody reuse is not recommended.
*Tips:
WB: For western blot detection of denatured protein samples. IHC: For immunohistochemical detection of paraffin sections (IHC-p) or frozen sections (IHC-f) of tissue samples. IF/ICC: For immunofluorescence detection of cell samples. ELISA(peptide): For ELISA detection of antigenic peptide.
Cite Format: Affinity Biosciences Cat# DF3708, RRID:AB_2836072.
Fold/Unfold
60S ribosomal protein L34; L34; MGC111005; Ribosomal protein L34;
Immunogens
A synthesized peptide derived from human RPL34, corresponding to a region within C-terminal amino acids.
- P49207 RL34_HUMAN:
- Protein BLAST With
- NCBI/
- ExPASy/
- Uniprot
MVQRLTYRRRLSYNTASNKTRLSRTPGNRIVYLYTKKVGKAPKSACGVCPGRLRGVRAVRPKVLMRLSKTKKHVSRAYGGSMCAKCVRDRIKRAFLIEEQKIVVKVLKAQAQSQKAK
Predictions
Score>80(red) has high confidence and is suggested to be used for WB detection. *The prediction model is mainly based on the alignment of immunogen sequences, the results are for reference only, not as the basis of quality assurance.
High(score>80) Medium(80>score>50) Low(score<50) No confidence
Research Backgrounds
Component of the large ribosomal subunit.
Cytoplasm>Cytosol. Cytoplasm. Endoplasmic reticulum.
Note: Detected on cytosolic polysomes (PubMed:25957688). Detected in ribosomes that are associated with the rough endoplasmic reticulum (By similarity).
Belongs to the eukaryotic ribosomal protein eL34 family.
Research Fields
· Genetic Information Processing > Translation > Ribosome.
References
Application: WB Species: Human Sample: DLD‐1 and HCT116 cells
Application: WB Species: Mouse Sample: BSR-T7/5 cells
Restrictive clause
Affinity Biosciences tests all products strictly. Citations are provided as a resource for additional applications that have not been validated by Affinity Biosciences. Please choose the appropriate format for each application and consult Materials and Methods sections for additional details about the use of any product in these publications.
For Research Use Only.
Not for use in diagnostic or therapeutic procedures. Not for resale. Not for distribution without written consent. Affinity Biosciences will not be held responsible for patent infringement or other violations that may occur with the use of our products. Affinity Biosciences, Affinity Biosciences Logo and all other trademarks are the property of Affinity Biosciences LTD.