RANKL Antibody - #AF0313
| Product: | RANKL Antibody |
| Catalog: | AF0313 |
| Description: | Rabbit polyclonal antibody to RANKL |
| Application: | WB IHC IF/ICC |
| Cited expt.: | WB, IHC, IF/ICC |
| Reactivity: | Human, Mouse, Rat |
| Prediction: | Pig, Bovine, Horse, Sheep, Rabbit, Dog |
| Mol.Wt.: | 40kDa; 35kD(Calculated). |
| Uniprot: | O14788 |
| RRID: | AB_2833477 |
Related Downloads
Protocols
Product Info
*The optimal dilutions should be determined by the end user. For optimal experimental results, antibody reuse is not recommended.
*Tips:
WB: For western blot detection of denatured protein samples. IHC: For immunohistochemical detection of paraffin sections (IHC-p) or frozen sections (IHC-f) of tissue samples. IF/ICC: For immunofluorescence detection of cell samples. ELISA(peptide): For ELISA detection of antigenic peptide.
Cite Format: Affinity Biosciences Cat# AF0313, RRID:AB_2833477.
Fold/Unfold
CD254; hRANKL2; ODF; OPGL; OPTB2; Osteoclast differentiation factor; Osteoprotegerin ligand; RANKL; Receptor activator of nuclear factor kappa B ligand; Receptor activator of nuclear factor kappa-B ligand; sOdf; TNF related activation induced cytokine; TNF-related activation-induced cytokine; TNF11_HUMAN; TNFSF 11; Tnfsf11; TRANCE; Tumor necrosis factor (ligand) superfamily member 11; Tumor necrosis factor ligand superfamily member 11; Tumor necrosis factor ligand superfamily member 11, soluble form;
Immunogens
A synthesized peptide derived from human RANKL, corresponding to a region within the internal amino acids.
Highest in the peripheral lymph nodes, weak in spleen, peripheral blood Leukocytes, bone marrow, heart, placenta, skeletal muscle, stomach and thyroid.
- O14788 TNF11_HUMAN:
- Protein BLAST With
- NCBI/
- ExPASy/
- Uniprot
MRRASRDYTKYLRGSEEMGGGPGAPHEGPLHAPPPPAPHQPPAASRSMFVALLGLGLGQVVCSVALFFYFRAQMDPNRISEDGTHCIYRILRLHENADFQDTTLESQDTKLIPDSCRRIKQAFQGAVQKELQHIVGSQHIRAEKAMVDGSWLDLAKRSKLEAQPFAHLTINATDIPSGSHKVSLSSWYHDRGWAKISNMTFSNGKLIVNQDGFYYLYANICFRHHETSGDLATEYLQLMVYVTKTSIKIPSSHTLMKGGSTKYWSGNSEFHFYSINVGGFFKLRSGEEISIEVSNPSLLDPDQDATYFGAFKVRDID
Predictions
Score>80(red) has high confidence and is suggested to be used for WB detection. *The prediction model is mainly based on the alignment of immunogen sequences, the results are for reference only, not as the basis of quality assurance.
High(score>80) Medium(80>score>50) Low(score<50) No confidence
Research Backgrounds
Cytokine that binds to TNFRSF11B/OPG and to TNFRSF11A/RANK. Osteoclast differentiation and activation factor. Augments the ability of dendritic cells to stimulate naive T-cell proliferation. May be an important regulator of interactions between T-cells and dendritic cells and may play a role in the regulation of the T-cell-dependent immune response. May also play an important role in enhanced bone-resorption in humoral hypercalcemia of malignancy. Induces osteoclastogenesis by activating multiple signaling pathways in osteoclast precursor cells, chief among which is induction of long lasting oscillations in the intracellular concentration of Ca (2+) resulting in the activation of NFATC1, which translocates to the nucleus and induces osteoclast-specific gene transcription to allow differentiation of osteoclasts. During osteoclast differentiation, in a TMEM64 and ATP2A2-dependent manner induces activation of CREB1 and mitochondrial ROS generation necessary for proper osteoclast generation (By similarity).
The soluble form of isoform 1 derives from the membrane form by proteolytic processing (By similarity). The cleavage may be catalyzed by ADAM17.
Cell membrane>Single-pass type II membrane protein.
Cell membrane>Single-pass type II membrane protein.
Cytoplasm.
Secreted.
Highest in the peripheral lymph nodes, weak in spleen, peripheral blood Leukocytes, bone marrow, heart, placenta, skeletal muscle, stomach and thyroid.
Belongs to the tumor necrosis factor family.
Research Fields
· Environmental Information Processing > Signaling molecules and interaction > Cytokine-cytokine receptor interaction. (View pathway)
· Environmental Information Processing > Signal transduction > NF-kappa B signaling pathway. (View pathway)
· Human Diseases > Cancers: Specific types > Breast cancer. (View pathway)
· Human Diseases > Immune diseases > Rheumatoid arthritis.
· Organismal Systems > Development > Osteoclast differentiation. (View pathway)
· Organismal Systems > Endocrine system > Prolactin signaling pathway. (View pathway)
References
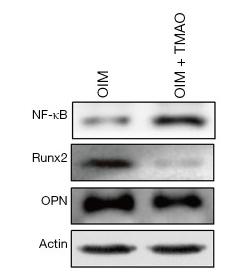
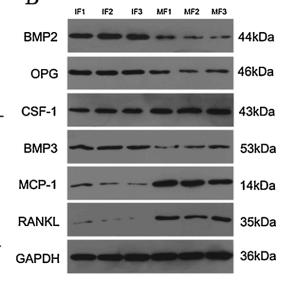
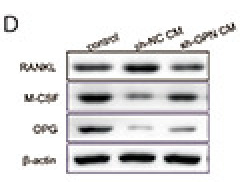
Application: WB Species: Mouse Sample:
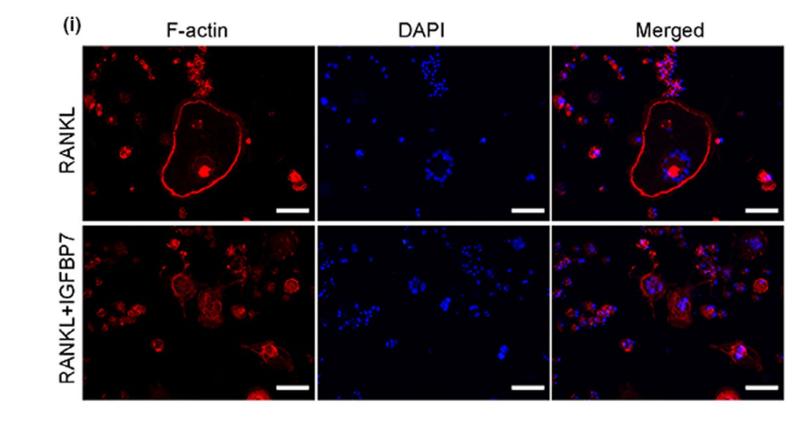
Application: IF/ICC Species: mice Sample: RAW264.7 cells
Application: IF/ICC Species: mouse Sample: osteoblastic cel
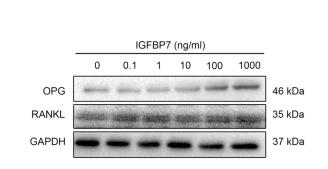
Application: WB Species: mouse Sample: MC3T3-E1
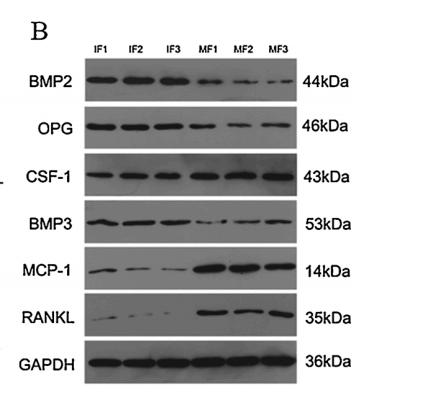
Application: WB Species: Rat Sample: osteoblasts
Restrictive clause
Affinity Biosciences tests all products strictly. Citations are provided as a resource for additional applications that have not been validated by Affinity Biosciences. Please choose the appropriate format for each application and consult Materials and Methods sections for additional details about the use of any product in these publications.
For Research Use Only.
Not for use in diagnostic or therapeutic procedures. Not for resale. Not for distribution without written consent. Affinity Biosciences will not be held responsible for patent infringement or other violations that may occur with the use of our products. Affinity Biosciences, Affinity Biosciences Logo and all other trademarks are the property of Affinity Biosciences LTD.