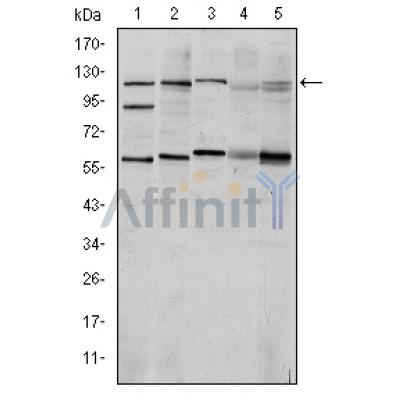
HIF1A Antibody - #BF0593
Product Info
*The optimal dilutions should be determined by the end user. For optimal experimental results, antibody reuse is not recommended.
*Tips:
WB: For western blot detection of denatured protein samples. IHC: For immunohistochemical detection of paraffin sections (IHC-p) or frozen sections (IHC-f) of tissue samples. IF/ICC: For immunofluorescence detection of cell samples. ELISA(peptide): For ELISA detection of antigenic peptide.
Cite Format: Affinity Biosciences Cat# BF0593, RRID:AB_2833903.
Fold/Unfold
ARNT interacting protein; ARNT-interacting protein; Basic helix loop helix PAS protein MOP1; Basic-helix-loop-helix-PAS protein MOP1; bHLHe78; Class E basic helix-loop-helix protein 78; HIF 1A; HIF 1alpha; HIF-1-alpha; HIF1 A; HIF1 Alpha; HIF1; HIF1-alpha; HIF1A; HIF1A_HUMAN; Hypoxia inducible factor 1 alpha; Hypoxia inducible factor 1 alpha isoform I.3; Hypoxia inducible factor 1 alpha subunit; Hypoxia inducible factor 1 alpha subunit basic helix loop helix transcription factor; Hypoxia inducible factor 1, alpha subunit (basic helix loop helix transcription factor); Hypoxia inducible factor1alpha; Hypoxia-inducible factor 1-alpha; Member of PAS protein 1; Member of PAS superfamily 1; Member of the PAS Superfamily 1; MOP 1; MOP1; PAS domain-containing protein 8; PASD 8; PASD8;
Immunogens
Purified recombinant fragment of human HIF1A expressed in E. Coli.
Expressed in most tissues with highest levels in kidney and heart. Overexpressed in the majority of common human cancers and their metastases, due to the presence of intratumoral hypoxia and as a result of mutations in genes encoding oncoproteins and tumor suppressors. A higher level expression seen in pituitary tumors as compared to the pituitary gland.
- Q16665 HIF1A_HUMAN:
- Protein BLAST With
- NCBI/
- ExPASy/
- Uniprot
MEGAGGANDKKKISSERRKEKSRDAARSRRSKESEVFYELAHQLPLPHNVSSHLDKASVMRLTISYLRVRKLLDAGDLDIEDDMKAQMNCFYLKALDGFVMVLTDDGDMIYISDNVNKYMGLTQFELTGHSVFDFTHPCDHEEMREMLTHRNGLVKKGKEQNTQRSFFLRMKCTLTSRGRTMNIKSATWKVLHCTGHIHVYDTNSNQPQCGYKKPPMTCLVLICEPIPHPSNIEIPLDSKTFLSRHSLDMKFSYCDERITELMGYEPEELLGRSIYEYYHALDSDHLTKTHHDMFTKGQVTTGQYRMLAKRGGYVWVETQATVIYNTKNSQPQCIVCVNYVVSGIIQHDLIFSLQQTECVLKPVESSDMKMTQLFTKVESEDTSSLFDKLKKEPDALTLLAPAAGDTIISLDFGSNDTETDDQQLEEVPLYNDVMLPSPNEKLQNINLAMSPLPTAETPKPLRSSADPALNQEVALKLEPNPESLELSFTMPQIQDQTPSPSDGSTRQSSPEPNSPSEYCFYVDSDMVNEFKLELVEKLFAEDTEAKNPFSTQDTDLDLEMLAPYIPMDDDFQLRSFDQLSPLESSSASPESASPQSTVTVFQQTQIQEPTANATTTTATTDELKTVTKDRMEDIKILIASPSPTHIHKETTSATSSPYRDTQSRTASPNRAGKGVIEQTEKSHPRSPNVLSVALSQRTTVPEEELNPKILALQNAQRKRKMEHDGSLFQAVGIGTLLQQPDDHAATTSLSWKRVKGCKSSEQNGMEQKTIILIPSDLACRLLGQSMDESGLPQLTSYDCEVNAPIQGSRNLLQGEELLRALDQVN
Research Backgrounds
Functions as a master transcriptional regulator of the adaptive response to hypoxia. Under hypoxic conditions, activates the transcription of over 40 genes, including erythropoietin, glucose transporters, glycolytic enzymes, vascular endothelial growth factor, HILPDA, and other genes whose protein products increase oxygen delivery or facilitate metabolic adaptation to hypoxia. Plays an essential role in embryonic vascularization, tumor angiogenesis and pathophysiology of ischemic disease. Heterodimerizes with ARNT; heterodimer binds to core DNA sequence 5'-TACGTG-3' within the hypoxia response element (HRE) of target gene promoters (By similarity). Activation requires recruitment of transcriptional coactivators such as CREBBP and EP300. Activity is enhanced by interaction with both, NCOA1 or NCOA2. Interaction with redox regulatory protein APEX seems to activate CTAD and potentiates activation by NCOA1 and CREBBP. Involved in the axonal distribution and transport of mitochondria in neurons during hypoxia.
S-nitrosylation of Cys-800 may be responsible for increased recruitment of p300 coactivator necessary for transcriptional activity of HIF-1 complex.
Requires phosphorylation for DNA-binding. Phosphorylation at Ser-247 by CSNK1D/CK1 represses kinase activity and impairs ARNT binding. Phosphorylation by GSK3-beta and PLK3 promote degradation by the proteasome.
Sumoylated; with SUMO1 under hypoxia. Sumoylation is enhanced through interaction with RWDD3. Both sumoylation and desumoylation seem to be involved in the regulation of its stability during hypoxia. Sumoylation can promote either its stabilization or its VHL-dependent degradation by promoting hydroxyproline-independent HIF1A-VHL complex binding, thus leading to HIF1A ubiquitination and proteasomal degradation. Desumoylation by SENP1 increases its stability amd transcriptional activity. There is a disaccord between various publications on the effect of sumoylation and desumoylation on its stability and transcriptional activity.
Acetylation of Lys-532 by ARD1 increases interaction with VHL and stimulates subsequent proteasomal degradation. Deacetylation of Lys-709 by SIRT2 increases its interaction with and hydroxylation by EGLN1 thereby inactivating HIF1A activity by inducing its proteasomal degradation.
Polyubiquitinated; in normoxia, following hydroxylation and interaction with VHL. Lys-532 appears to be the principal site of ubiquitination. Clioquinol, the Cu/Zn-chelator, inhibits ubiquitination through preventing hydroxylation at Asn-803. Ubiquitinated by a CUL2-based E3 ligase.
In normoxia, is hydroxylated on Pro-402 and Pro-564 in the oxygen-dependent degradation domain (ODD) by EGLN1/PHD2 and EGLN2/PHD1. EGLN3/PHD3 has also been shown to hydroxylate Pro-564. The hydroxylated prolines promote interaction with VHL, initiating rapid ubiquitination and subsequent proteasomal degradation. Deubiquitinated by USP20. Under hypoxia, proline hydroxylation is impaired and ubiquitination is attenuated, resulting in stabilization. In normoxia, is hydroxylated on Asn-803 by HIF1AN, thus abrogating interaction with CREBBP and EP300 and preventing transcriptional activation. This hydroxylation is inhibited by the Cu/Zn-chelator, Clioquinol. Repressed by iron ion, via Fe(2+) prolyl hydroxylase (PHD) enzymes-mediated hydroxylation and subsequent proteasomal degradation.
The iron and 2-oxoglutarate dependent 3-hydroxylation of asparagine is (S) stereospecific within HIF CTAD domains.
Cytoplasm. Nucleus. Nucleus speckle.
Note: Colocalizes with HIF3A in the nucleus and speckles (By similarity). Cytoplasmic in normoxia, nuclear translocation in response to hypoxia (PubMed:9822602).
Expressed in most tissues with highest levels in kidney and heart. Overexpressed in the majority of common human cancers and their metastases, due to the presence of intratumoral hypoxia and as a result of mutations in genes encoding oncoproteins and tumor suppressors. A higher level expression seen in pituitary tumors as compared to the pituitary gland.
Contains two independent C-terminal transactivation domains, NTAD and CTAD, which function synergistically. Their transcriptional activity is repressed by an intervening inhibitory domain (ID).
Research Fields
· Cellular Processes > Transport and catabolism > Autophagy - animal. (View pathway)
· Environmental Information Processing > Signal transduction > HIF-1 signaling pathway. (View pathway)
· Human Diseases > Cancers: Overview > Pathways in cancer. (View pathway)
· Human Diseases > Cancers: Overview > Proteoglycans in cancer.
· Human Diseases > Cancers: Specific types > Renal cell carcinoma. (View pathway)
· Human Diseases > Cancers: Overview > Central carbon metabolism in cancer. (View pathway)
· Human Diseases > Cancers: Overview > Choline metabolism in cancer. (View pathway)
· Organismal Systems > Immune system > Th17 cell differentiation. (View pathway)
· Organismal Systems > Endocrine system > Thyroid hormone signaling pathway. (View pathway)
References
Application: WB Species: Human Sample: HCC tissues
Application: WB Species: human Sample: breast cell
Application: WB Species: Mice Sample: kidney tissues
Restrictive clause
Affinity Biosciences tests all products strictly. Citations are provided as a resource for additional applications that have not been validated by Affinity Biosciences. Please choose the appropriate format for each application and consult Materials and Methods sections for additional details about the use of any product in these publications.
For Research Use Only.
Not for use in diagnostic or therapeutic procedures. Not for resale. Not for distribution without written consent. Affinity Biosciences will not be held responsible for patent infringement or other violations that may occur with the use of our products. Affinity Biosciences, Affinity Biosciences Logo and all other trademarks are the property of Affinity Biosciences LTD.