AFfirm™ NLRP3 Mouse monoclonal Antibody - #BF8029
Product Info
*The optimal dilutions should be determined by the end user. For optimal experimental results, antibody reuse is not recommended.
*Tips:
WB: For western blot detection of denatured protein samples. IHC: For immunohistochemical detection of paraffin sections (IHC-p) or frozen sections (IHC-f) of tissue samples. IF/ICC: For immunofluorescence detection of cell samples. ELISA(peptide): For ELISA detection of antigenic peptide.
Fold/Unfold
AGTAVPRL; AII/AVP; Angiotensin/vasopressin receptor AII/AVP like; Angiotensin/vasopressin receptor AII/AVP-like; C1orf7; Caterpiller protein 1.1; CIAS 1; CIAS1; CLR1.1; Cold autoinflammatory syndrome 1; Cold autoinflammatory syndrome 1 protein; Cryopyrin; Familial cold autoinflammatory syndrome; FCAS; FCU; LRR and PYD domains-containing protein 3; Muckle-Wells syndrome; MWS; NACHT; NACHT LRR and PYD containing protein 3; NALP 3; NALP3; NALP3_HUMAN; NLR family pyrin domain containing 3; NLRP3; PYPAF 1; PYPAF1; PYRIN containing APAF1 like protein 1; PYRIN-containing APAF1-like protein 1;
Immunogens
A synthesized peptide derived from Human NLRP3.
Predominantly expressed in macrophages. Also expressed in dendritic cells, B- and T-cells (at protein level) (PubMed:11786556) (PubMed:17164409). Expressed in LPS-treated granulocytes, but not in resting cells (at protein level) (PubMed:17164409). Expression in monocytes is very weak (at protein level) (PubMed:17164409). Expressed in stratified non-keratinizing squamous epithelium, including oral, esophageal and ectocervical mucosa and in the Hassall's corpuscles in the thymus. Also, detected in the stratified epithelium covering the bladder and ureter (transitional mucosa) (at protein level) (PubMed:17164409). Expressed in lung epithelial cells (at protein level) (PubMed:23229815). Expressed in chondrocytes (PubMed:12032915). Expressed at low levels in resting osteoblasts (PubMed:17907925).
- Q96P20 NLRP3_HUMAN:
- Protein BLAST With
- NCBI/
- ExPASy/
- Uniprot
MKMASTRCKLARYLEDLEDVDLKKFKMHLEDYPPQKGCIPLPRGQTEKADHVDLATLMIDFNGEEKAWAMAVWIFAAINRRDLYEKAKRDEPKWGSDNARVSNPTVICQEDSIEEEWMGLLEYLSRISICKMKKDYRKKYRKYVRSRFQCIEDRNARLGESVSLNKRYTRLRLIKEHRSQQEREQELLAIGKTKTCESPVSPIKMELLFDPDDEHSEPVHTVVFQGAAGIGKTILARKMMLDWASGTLYQDRFDYLFYIHCREVSLVTQRSLGDLIMSCCPDPNPPIHKIVRKPSRILFLMDGFDELQGAFDEHIGPLCTDWQKAERGDILLSSLIRKKLLPEASLLITTRPVALEKLQHLLDHPRHVEILGFSEAKRKEYFFKYFSDEAQARAAFSLIQENEVLFTMCFIPLVCWIVCTGLKQQMESGKSLAQTSKTTTAVYVFFLSSLLQPRGGSQEHGLCAHLWGLCSLAADGIWNQKILFEESDLRNHGLQKADVSAFLRMNLFQKEVDCEKFYSFIHMTFQEFFAAMYYLLEEEKEGRTNVPGSRLKLPSRDVTVLLENYGKFEKGYLIFVVRFLFGLVNQERTSYLEKKLSCKISQQIRLELLKWIEVKAKAKKLQIQPSQLELFYCLYEMQEEDFVQRAMDYFPKIEINLSTRMDHMVSSFCIENCHRVESLSLGFLHNMPKEEEEEEKEGRHLDMVQCVLPSSSHAACSHGLVNSHLTSSFCRGLFSVLSTSQSLTELDLSDNSLGDPGMRVLCETLQHPGCNIRRLWLGRCGLSHECCFDISLVLSSNQKLVELDLSDNALGDFGIRLLCVGLKHLLCNLKKLWLVSCCLTSACCQDLASVLSTSHSLTRLYVGENALGDSGVAILCEKAKNPQCNLQKLGLVNSGLTSVCCSALSSVLSTNQNLTHLYLRGNTLGDKGIKLLCEGLLHPDCKLQVLELDNCNLTSHCCWDLSTLLTSSQSLRKLSLGNNDLGDLGVMMFCEVLKQQSCLLQNLGLSEMYFNYETKSALETLQEEKPELTVVFEPSW
Research Backgrounds
As the sensor component of the NLRP3 inflammasome, plays a crucial role in innate immunity and inflammation. In response to pathogens and other damage-associated signals, initiates the formation of the inflammasome polymeric complex, made of NLRP3, PYCARD and CASP1 (and possibly CASP4 and CASP5). Recruitment of proCASP1 to the inflammasome promotes its activation and CASP1-catalyzed IL1B and IL18 maturation and secretion in the extracellular milieu. Activation of NLRP3 inflammasome is also required for HMGB1 secretion. The active cytokines and HMGB1 stimulate inflammatory responses. Inflammasomes can also induce pyroptosis, an inflammatory form of programmed cell death. Under resting conditions, NLRP3 is autoinhibited. NLRP3 activation stimuli include extracellular ATP, reactive oxygen species, K(+) efflux, crystals of monosodium urate or cholesterol, amyloid-beta fibers, environmental or industrial particles and nanoparticles, cytosolic dsRNA, etc. However, it is unclear what constitutes the direct NLRP3 activator. Activation in presence of cytosolic dsRNA is mediated by DHX33. Independently of inflammasome activation, regulates the differentiation of T helper 2 (Th2) cells and has a role in Th2 cell-dependent asthma and tumor growth (By similarity). During Th2 differentiation, required for optimal IRF4 binding to IL4 promoter and for IRF4-dependent IL4 transcription. Binds to the consensus DNA sequence 5'-GRRGGNRGAG-3'. May also participate in the transcription of IL5, IL13, GATA3, CCR3, CCR4 and MAF (By similarity).
The disulfide bond in the pyrin domain might play a role in reactive oxygen species-mediated activation.
Ubiquitinated; undergoes both 'Lys-48'- and 'Lys-63'-linked polyubiquitination. Ubiquitination does not lead to degradation, but inhibits inflammasome activation (By similarity). Deubiquitination is catalyzed by BRCC3 and associated with NLRP3 activation and inflammasome assembly. This process can be induced by the activation of Toll-like receptors (by LPS), through a non-transcriptional pathway dependent on the mitochondrial production of reactive oxygen species, and by ATP.
Cytoplasm>Cytosol. Inflammasome. Endoplasmic reticulum. Secreted. Nucleus.
Note: In macrophages, under resting conditions, mainly located in the cytosol, on the endoplasmic reticulum. After stimulation with inducers of the NLRP3 inflammasome, mitochondria redistribute in the vicinity of the endoplasmic reticulum in the perinuclear region, which results in colocalization of NLRP3 on the endoplasmic reticulum and PYCARD on mitochondria, allowing the activation of inflammasome assembly. After the induction of pyroptosis, inflammasome specks are released into the extracellular space where they can further promote IL1B processing and where they can be engulfed by macrophages. Phagocytosis induces lysosomal damage and inflammasome activation in the recipient cells (PubMed:24952504). In the Th2 subset of CD4(+) helper T-cells, mainly located in the nucleus. Nuclear localization depends upon KPNA2. In the Th1 subset of CD4(+) helper T-cells, mainly cytoplasmic (By similarity).
Golgi apparatus membrane.
Note: (Microbial infection) Upon HRSV infection, the protein is mainly located in lipid rafts in the Golgi membrane.
Predominantly expressed in macrophages. Also expressed in dendritic cells, B- and T-cells (at protein level). Expressed in LPS-treated granulocytes, but not in resting cells (at protein level). Expression in monocytes is very weak (at protein level). Expressed in stratified non-keratinizing squamous epithelium, including oral, esophageal and ectocervical mucosa and in the Hassall's corpuscles in the thymus. Also, detected in the stratified epithelium covering the bladder and ureter (transitional mucosa) (at protein level). Expressed in lung epithelial cells (at protein level). Expressed in chondrocytes. Expressed at low levels in resting osteoblasts.
The pyrin domain (also called DAPIN domain or PYD) is involved in PYCARD-binding.
The LRR domain mediates the interaction with IRF4 and PML.
Intramolecular interactions between NACHT and leucine-rich repeat (LRR) domains may be important for autoinhibition in the absence of activating signal.
Belongs to the NLRP family.
Research Fields
· Cellular Processes > Cell growth and death > Necroptosis. (View pathway)
· Human Diseases > Infectious diseases: Bacterial > Pertussis.
· Human Diseases > Infectious diseases: Viral > Influenza A.
· Organismal Systems > Immune system > NOD-like receptor signaling pathway. (View pathway)
References
Application: WB Species: Human Sample: HVSMCs
Application: IF/ICC Species: Mouse Sample:
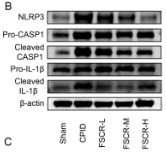
Application: WB Species: Rat Sample: spinal cord
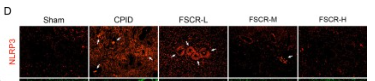
Application: IF/ICC Species: Rat Sample: spinal cord
Restrictive clause
Affinity Biosciences tests all products strictly. Citations are provided as a resource for additional applications that have not been validated by Affinity Biosciences. Please choose the appropriate format for each application and consult Materials and Methods sections for additional details about the use of any product in these publications.
For Research Use Only.
Not for use in diagnostic or therapeutic procedures. Not for resale. Not for distribution without written consent. Affinity Biosciences will not be held responsible for patent infringement or other violations that may occur with the use of our products. Affinity Biosciences, Affinity Biosciences Logo and all other trademarks are the property of Affinity Biosciences LTD.