MMP7 Antibody - #AF0218
| Product: | MMP7 Antibody |
| Catalog: | AF0218 |
| Description: | Rabbit polyclonal antibody to MMP7 |
| Application: | WB IHC IF/ICC |
| Reactivity: | Human, Mouse, Rat |
| Prediction: | Pig, Sheep, Rabbit |
| Mol.Wt.: | 29kDa; 30kD(Calculated). |
| Uniprot: | P09237 |
| RRID: | AB_2833348 |
Related Downloads
Protocols
Product Info
*The optimal dilutions should be determined by the end user.
*Tips:
WB: For western blot detection of denatured protein samples. IHC: For immunohistochemical detection of paraffin sections (IHC-p) or frozen sections (IHC-f) of tissue samples. IF/ICC: For immunofluorescence detection of cell samples. ELISA(peptide): For ELISA detection of antigenic peptide.
Cite Format: Affinity Biosciences Cat# AF0218, RRID:AB_2833348.
Fold/Unfold
Matrilysin; Matrin; Matrix Metalloproteinase 7; Matrix metalloproteinase-7; MMP 7; MMP-7; MMP7; MMP7_HUMAN; MPSL1; PUMP 1; Pump 1 protease; Pump-1 protease; PUMP1; Uterine matrilysin; Uterine metalloproteinase;
Immunogens
- P09237 MMP7_HUMAN:
- Protein BLAST With
- NCBI/
- ExPASy/
- Uniprot
MRLTVLCAVCLLPGSLALPLPQEAGGMSELQWEQAQDYLKRFYLYDSETKNANSLEAKLKEMQKFFGLPITGMLNSRVIEIMQKPRCGVPDVAEYSLFPNSPKWTSKVVTYRIVSYTRDLPHITVDRLVSKALNMWGKEIPLHFRKVVWGTADIMIGFARGAHGDSYPFDGPGNTLAHAFAPGTGLGGDAHFDEDERWTDGSSLGINFLYAATHELGHSLGMGHSSDPNAVMYPTYGNGDPQNFKLSQDDIKGIQKLYGKRSNSRKK
Predictions
Score>80(red) has high confidence and is suggested to be used for WB detection. *The prediction model is mainly based on the alignment of immunogen sequences, the results are for reference only, not as the basis of quality assurance.
High(score>80) Medium(80>score>50) Low(score<50) No confidence
PTMs - P09237 As Substrate
| Site | PTM Type | Enzyme | Source |
|---|---|---|---|
| S47 | Phosphorylation | Uniprot | |
| T49 | Phosphorylation | Uniprot | |
| K84 | Acetylation | Uniprot |
Research Backgrounds
Degrades casein, gelatins of types I, III, IV, and V, and fibronectin. Activates procollagenase.
Secreted>Extracellular space>Extracellular matrix.
The conserved cysteine present in the cysteine-switch motif binds the catalytic zinc ion, thus inhibiting the enzyme. The dissociation of the cysteine from the zinc ion upon the activation-peptide release activates the enzyme.
Belongs to the peptidase M10A family.
Research Fields
· Environmental Information Processing > Signal transduction > Wnt signaling pathway. (View pathway)
References
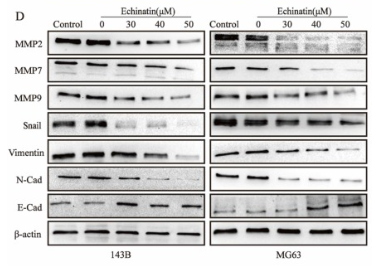
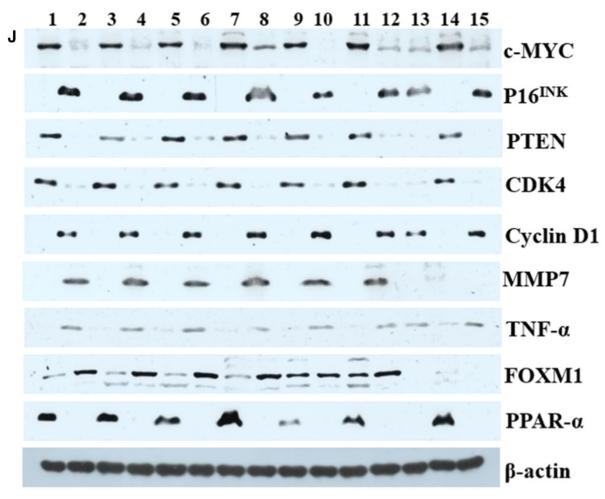
Application: WB Species: Human Sample: OS cells
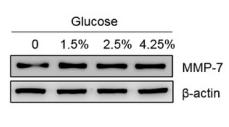
Application: WB Species: Human Sample: BC cells
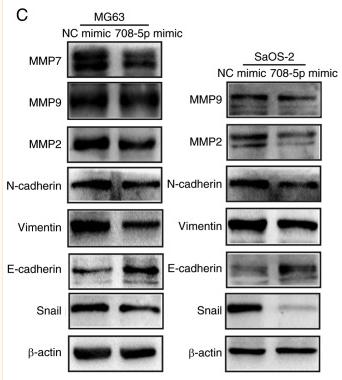
Application: WB Species: Human Sample: peritoneal mesothelial cells
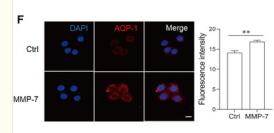
Application: IF/ICC Species: Human Sample: peritoneal mesothelial cells
Application: IHC Species: Rat Sample: kidneys
Application: WB Species: Human Sample: OS cells
Application: WB Species: Mice Sample: tumor tissues
Restrictive clause
Affinity Biosciences tests all products strictly. Citations are provided as a resource for additional applications that have not been validated by Affinity Biosciences. Please choose the appropriate format for each application and consult Materials and Methods sections for additional details about the use of any product in these publications.
For Research Use Only.
Not for use in diagnostic or therapeutic procedures. Not for resale. Not for distribution without written consent. Affinity Biosciences will not be held responsible for patent infringement or other violations that may occur with the use of our products. Affinity Biosciences, Affinity Biosciences Logo and all other trademarks are the property of Affinity Biosciences LTD.