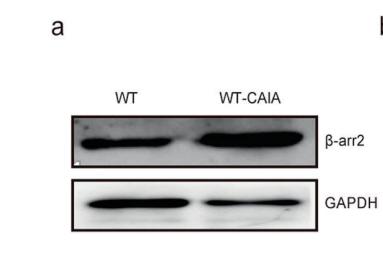
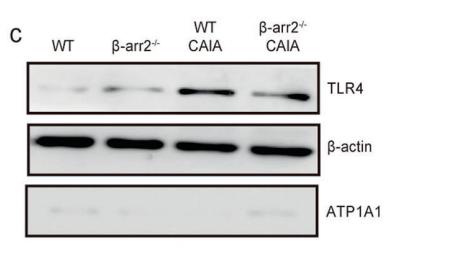
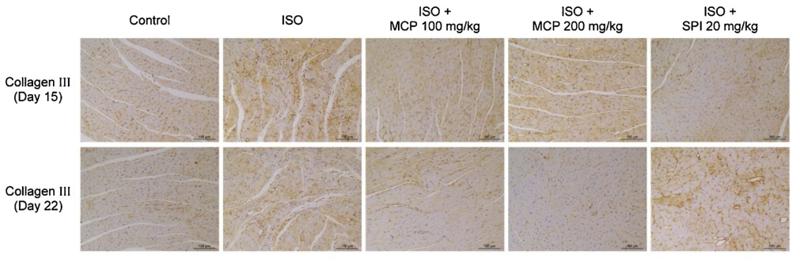
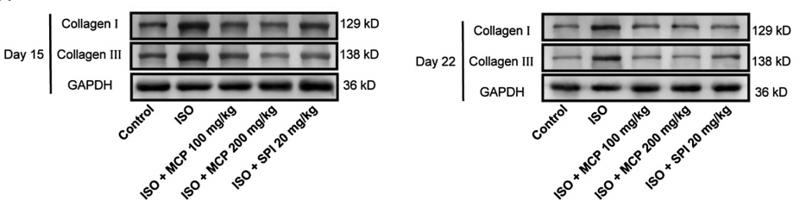
Collagen III Antibody - #AF0136
| Product: | Collagen III Antibody |
| Catalog: | AF0136 |
| Description: | Rabbit polyclonal antibody to Collagen III |
| Application: | WB IHC IF/ICC |
| Reactivity: | Human, Mouse, Rat |
| Prediction: | Pig, Bovine, Horse, Sheep, Rabbit, Dog, Chicken, Xenopus |
| Mol.Wt.: | 138kDa; 139kD(Calculated). |
| Uniprot: | P02461 |
| RRID: | AB_2813770 |
Product Info
*The optimal dilutions should be determined by the end user.
*Tips:
WB: For western blot detection of denatured protein samples. IHC: For immunohistochemical detection of paraffin sections (IHC-p) or frozen sections (IHC-f) of tissue samples. IF/ICC: For immunofluorescence detection of cell samples. ELISA(peptide): For ELISA detection of antigenic peptide.
Cite Format: Affinity Biosciences Cat# AF0136, RRID:AB_2813770.
Fold/Unfold
Alpha 1 type III collagen; Alpha1 (III) collagen; CO3A1_HUMAN; COL 3A1; COL3A1; Collagen alpha 1(III) chain; Collagen alpha-1(III) chain; Collagen III alpha 1 chain precursor; Collagen III alpha 1 polypeptide; Collagen type III alpha 1 (Ehlers Danlos syndrome type IV autosomal dominant); Collagen type III alpha 1; Collagen type III alpha 1 chain; Collagen type III alpha; Collagen, fetal; EDS4A; Ehlers Danlos syndrome type IV, autosomal dominant; Fetal collagen; Type III collagen;
Immunogens
- P02461 CO3A1_HUMAN:
- Protein BLAST With
- NCBI/
- ExPASy/
- Uniprot
MMSFVQKGSWLLLALLHPTIILAQQEAVEGGCSHLGQSYADRDVWKPEPCQICVCDSGSVLCDDIICDDQELDCPNPEIPFGECCAVCPQPPTAPTRPPNGQGPQGPKGDPGPPGIPGRNGDPGIPGQPGSPGSPGPPGICESCPTGPQNYSPQYDSYDVKSGVAVGGLAGYPGPAGPPGPPGPPGTSGHPGSPGSPGYQGPPGEPGQAGPSGPPGPPGAIGPSGPAGKDGESGRPGRPGERGLPGPPGIKGPAGIPGFPGMKGHRGFDGRNGEKGETGAPGLKGENGLPGENGAPGPMGPRGAPGERGRPGLPGAAGARGNDGARGSDGQPGPPGPPGTAGFPGSPGAKGEVGPAGSPGSNGAPGQRGEPGPQGHAGAQGPPGPPGINGSPGGKGEMGPAGIPGAPGLMGARGPPGPAGANGAPGLRGGAGEPGKNGAKGEPGPRGERGEAGIPGVPGAKGEDGKDGSPGEPGANGLPGAAGERGAPGFRGPAGPNGIPGEKGPAGERGAPGPAGPRGAAGEPGRDGVPGGPGMRGMPGSPGGPGSDGKPGPPGSQGESGRPGPPGPSGPRGQPGVMGFPGPKGNDGAPGKNGERGGPGGPGPQGPPGKNGETGPQGPPGPTGPGGDKGDTGPPGPQGLQGLPGTGGPPGENGKPGEPGPKGDAGAPGAPGGKGDAGAPGERGPPGLAGAPGLRGGAGPPGPEGGKGAAGPPGPPGAAGTPGLQGMPGERGGLGSPGPKGDKGEPGGPGADGVPGKDGPRGPTGPIGPPGPAGQPGDKGEGGAPGLPGIAGPRGSPGERGETGPPGPAGFPGAPGQNGEPGGKGERGAPGEKGEGGPPGVAGPPGGSGPAGPPGPQGVKGERGSPGGPGAAGFPGARGLPGPPGSNGNPGPPGPSGSPGKDGPPGPAGNTGAPGSPGVSGPKGDAGQPGEKGSPGAQGPPGAPGPLGIAGITGARGLAGPPGMPGPRGSPGPQGVKGESGKPGANGLSGERGPPGPQGLPGLAGTAGEPGRDGNPGSDGLPGRDGSPGGKGDRGENGSPGAPGAPGHPGPPGPVGPAGKSGDRGESGPAGPAGAPGPAGSRGAPGPQGPRGDKGETGERGAAGIKGHRGFPGNPGAPGSPGPAGQQGAIGSPGPAGPRGPVGPSGPPGKDGTSGHPGPIGPPGPRGNRGERGSEGSPGHPGQPGPPGPPGAPGPCCGGVGAAAIAGIGGEKAGGFAPYYGDEPMDFKINTDEIMTSLKSVNGQIESLISPDGSRKNPARNCRDLKFCHPELKSGEYWVDPNQGCKLDAIKVFCNMETGETCISANPLNVPRKHWWTDSSAEKKHVWFGESMDGGFQFSYGNPELPEDVLDVHLAFLRLLSSRASQNITYHCKNSIAYMDQASGNVKKALKLMGSNEGEFKAEGNSKFTYTVLEDGCTKHTGEWSKTVFEYRTRKAVRLPIVDIAPYDIGGPDQEFGVDVGPVCFL
Predictions
Score>80(red) has high confidence and is suggested to be used for WB detection. *The prediction model is mainly based on the alignment of immunogen sequences, the results are for reference only, not as the basis of quality assurance.
High(score>80) Medium(80>score>50) Low(score<50) No confidence
PTMs - P02461 As Substrate
| Site | PTM Type | Enzyme | Source |
|---|---|---|---|
| S143 | Phosphorylation | Uniprot | |
| K757 | Ubiquitination | Uniprot | |
| S916 | Phosphorylation | Uniprot | |
| S1145 | O-Glycosylation | Uniprot | |
| Y1220 | Phosphorylation | Uniprot | |
| S1237 | Phosphorylation | Uniprot | |
| Y1370 | Phosphorylation | Uniprot | |
| Y1378 | Phosphorylation | Uniprot | |
| S1383 | Phosphorylation | Uniprot | |
| K1387 | Acetylation | Uniprot |
Research Backgrounds
Collagen type III occurs in most soft connective tissues along with type I collagen. Involved in regulation of cortical development. Is the major ligand of ADGRG1 in the developing brain and binding to ADGRG1 inhibits neuronal migration and activates the RhoA pathway by coupling ADGRG1 to GNA13 and possibly GNA12.
Proline residues at the third position of the tripeptide repeating unit (G-X-Y) are hydroxylated in some or all of the chains.
O-linked glycan consists of a Glc-Gal disaccharide bound to the oxygen atom of a post-translationally added hydroxyl group.
Secreted>Extracellular space>Extracellular matrix.
Trimers of identical alpha 1(III) chains. The chains are linked to each other by interchain disulfide bonds. Trimers are also cross-linked via hydroxylysines. Interacts with ADGRG1.
The C-terminal propeptide, also known as COLFI domain, have crucial roles in tissue growth and repair by controlling both the intracellular assembly of procollagen molecules and the extracellular assembly of collagen fibrils. It binds a calcium ion which is essential for its function.
Belongs to the fibrillar collagen family.
Research Fields
· Human Diseases > Infectious diseases: Parasitic > Amoebiasis.
· Organismal Systems > Immune system > Platelet activation. (View pathway)
· Organismal Systems > Endocrine system > Relaxin signaling pathway.
· Organismal Systems > Digestive system > Protein digestion and absorption.
References
Application: IF/ICC Species: rat Sample: tendon
Application: IHC Species: rat Sample: tendon
Application: IHC Species: rat Sample: heart
Application: WB Species: rat Sample: heart
Application: WB Species: Human Sample: HSFs
Application: WB Species: mouse Sample: heart
Application: IF/ICC Species: mouse Sample: CFs
Application: WB Species: Mouse Sample: myocardial tissues
Application: WB Species: Mice Sample: lung tissues
Restrictive clause
Affinity Biosciences tests all products strictly. Citations are provided as a resource for additional applications that have not been validated by Affinity Biosciences. Please choose the appropriate format for each application and consult Materials and Methods sections for additional details about the use of any product in these publications.
For Research Use Only.
Not for use in diagnostic or therapeutic procedures. Not for resale. Not for distribution without written consent. Affinity Biosciences will not be held responsible for patent infringement or other violations that may occur with the use of our products. Affinity Biosciences, Affinity Biosciences Logo and all other trademarks are the property of Affinity Biosciences LTD.