Phospho-Cdk1/2 (Thr14) Antibody - #DF2944
| Product: | Phospho-Cdk1/2 (Thr14) Antibody |
| Catalog: | DF2944 |
| Description: | Rabbit polyclonal antibody to Phospho-Cdk1/2 (Thr14) |
| Application: | WB IHC |
| Cited expt.: | WB |
| Reactivity: | Human, Mouse, Rat |
| Prediction: | Pig, Zebrafish, Bovine, Horse, Rabbit, Dog, Chicken, Xenopus |
| Mol.Wt.: | 34kDa; 34kD(Calculated). |
| Uniprot: | P06493 | P24941 |
| RRID: | AB_2840928 |
Product Info
*The optimal dilutions should be determined by the end user. For optimal experimental results, antibody reuse is not recommended.
*Tips:
WB: For western blot detection of denatured protein samples. IHC: For immunohistochemical detection of paraffin sections (IHC-p) or frozen sections (IHC-f) of tissue samples. IF/ICC: For immunofluorescence detection of cell samples. ELISA(peptide): For ELISA detection of antigenic peptide.
Cite Format: Affinity Biosciences Cat# DF2944, RRID:AB_2840928.
Fold/Unfold
Cdc 2; Cdc2; CDC28A; CDK 1; CDK1; CDK1_HUMAN; CDKN1; CELL CYCLE CONTROLLER CDC2; Cell division control protein 2; Cell division control protein 2 homolog; Cell division cycle 2 G1 to S and G2 to M; Cell division protein kinase 1; Cell Divsion Cycle 2 Protein; Cyclin Dependent Kinase 1; Cyclin-dependent kinase 1; DKFZp686L20222; MGC111195; p34 Cdk1; p34 protein kinase; P34CDC2; Cdc2 related protein kinase; cdc2-related protein kinase; CDC28; CDC2A; Cdk 2; CDK1; CDK2; CDK2_HUMAN; CDKN2; Cell devision kinase 2; Cell division protein kinase 2; Cyclin dependent kinase 2; cyclin dependent kinase 2-alpha; Cyclin-dependent kinase 2; kinase Cdc2; MPF; p33 protein kinase; p33(CDK2);
Immunogens
A synthesized peptide derived from human Cdk1/2 around the phosphorylation site of T14.
- P06493 CDK1_HUMAN:
- Protein BLAST With
- NCBI/
- ExPASy/
- Uniprot
MEDYTKIEKIGEGTYGVVYKGRHKTTGQVVAMKKIRLESEEEGVPSTAIREISLLKELRHPNIVSLQDVLMQDSRLYLIFEFLSMDLKKYLDSIPPGQYMDSSLVKSYLYQILQGIVFCHSRRVLHRDLKPQNLLIDDKGTIKLADFGLARAFGIPIRVYTHEVVTLWYRSPEVLLGSARYSTPVDIWSIGTIFAELATKKPLFHGDSEIDQLFRIFRALGTPNNEVWPEVESLQDYKNTFPKWKPGSLASHVKNLDENGLDLLSKMLIYDPAKRISGKMALNHPYFNDLDNQIKKM
- P24941 CDK2_HUMAN:
- Protein BLAST With
- NCBI/
- ExPASy/
- Uniprot
MENFQKVEKIGEGTYGVVYKARNKLTGEVVALKKIRLDTETEGVPSTAIREISLLKELNHPNIVKLLDVIHTENKLYLVFEFLHQDLKKFMDASALTGIPLPLIKSYLFQLLQGLAFCHSHRVLHRDLKPQNLLINTEGAIKLADFGLARAFGVPVRTYTHEVVTLWYRAPEILLGCKYYSTAVDIWSLGCIFAEMVTRRALFPGDSEIDQLFRIFRTLGTPDEVVWPGVTSMPDYKPSFPKWARQDFSKVVPPLDEDGRSLLSQMLHYDPNKRISAKAALAHPFFQDVTKPVPHLRL
Predictions
Score>80(red) has high confidence and is suggested to be used for WB detection. *The prediction model is mainly based on the alignment of immunogen sequences, the results are for reference only, not as the basis of quality assurance.
High(score>80) Medium(80>score>50) Low(score<50) No confidence
Research Backgrounds
Plays a key role in the control of the eukaryotic cell cycle by modulating the centrosome cycle as well as mitotic onset; promotes G2-M transition, and regulates G1 progress and G1-S transition via association with multiple interphase cyclins. Required in higher cells for entry into S-phase and mitosis. Phosphorylates PARVA/actopaxin, APC, AMPH, APC, BARD1, Bcl-xL/BCL2L1, BRCA2, CALD1, CASP8, CDC7, CDC20, CDC25A, CDC25C, CC2D1A, CENPA, CSNK2 proteins/CKII, FZR1/CDH1, CDK7, CEBPB, CHAMP1, DMD/dystrophin, EEF1 proteins/EF-1, EZH2, KIF11/EG5, EGFR, FANCG, FOS, GFAP, GOLGA2/GM130, GRASP1, UBE2A/hHR6A, HIST1H1 proteins/histone H1, HMGA1, HIVEP3/KRC, LMNA, LMNB, LMNC, LBR, LATS1, MAP1B, MAP4, MARCKS, MCM2, MCM4, MKLP1, MYB, NEFH, NFIC, NPC/nuclear pore complex, PITPNM1/NIR2, NPM1, NCL, NUCKS1, NPM1/numatrin, ORC1, PRKAR2A, EEF1E1/p18, EIF3F/p47, p53/TP53, NONO/p54NRB, PAPOLA, PLEC/plectin, RB1, TPPP, UL40/R2, RAB4A, RAP1GAP, RCC1, RPS6KB1/S6K1, KHDRBS1/SAM68, ESPL1, SKI, BIRC5/survivin, STIP1, TEX14, beta-tubulins, MAPT/TAU, NEDD1, VIM/vimentin, TK1, FOXO1, RUNX1/AML1, SAMHD1, SIRT2 and RUNX2. CDK1/CDC2-cyclin-B controls pronuclear union in interphase fertilized eggs. Essential for early stages of embryonic development. During G2 and early mitosis, CDC25A/B/C-mediated dephosphorylation activates CDK1/cyclin complexes which phosphorylate several substrates that trigger at least centrosome separation, Golgi dynamics, nuclear envelope breakdown and chromosome condensation. Once chromosomes are condensed and aligned at the metaphase plate, CDK1 activity is switched off by WEE1- and PKMYT1-mediated phosphorylation to allow sister chromatid separation, chromosome decondensation, reformation of the nuclear envelope and cytokinesis. Inactivated by PKR/EIF2AK2- and WEE1-mediated phosphorylation upon DNA damage to stop cell cycle and genome replication at the G2 checkpoint thus facilitating DNA repair. Reactivated after successful DNA repair through WIP1-dependent signaling leading to CDC25A/B/C-mediated dephosphorylation and restoring cell cycle progression. In proliferating cells, CDK1-mediated FOXO1 phosphorylation at the G2-M phase represses FOXO1 interaction with 14-3-3 proteins and thereby promotes FOXO1 nuclear accumulation and transcription factor activity, leading to cell death of postmitotic neurons. The phosphorylation of beta-tubulins regulates microtubule dynamics during mitosis. NEDD1 phosphorylation promotes PLK1-mediated NEDD1 phosphorylation and subsequent targeting of the gamma-tubulin ring complex (gTuRC) to the centrosome, an important step for spindle formation. In addition, CC2D1A phosphorylation regulates CC2D1A spindle pole localization and association with SCC1/RAD21 and centriole cohesion during mitosis. The phosphorylation of Bcl-xL/BCL2L1 after prolongated G2 arrest upon DNA damage triggers apoptosis. In contrast, CASP8 phosphorylation during mitosis prevents its activation by proteolysis and subsequent apoptosis. This phosphorylation occurs in cancer cell lines, as well as in primary breast tissues and lymphocytes. EZH2 phosphorylation promotes H3K27me3 maintenance and epigenetic gene silencing. CALD1 phosphorylation promotes Schwann cell migration during peripheral nerve regeneration. CDK1-cyclin-B complex phosphorylates NCKAP5L and mediates its dissociation from centrosomes during mitosis. Regulates the amplitude of the cyclic expression of the core clock gene ARNTL/BMAL1 by phosphorylating its transcriptional repressor NR1D1, and this phosphorylation is necessary for SCF(FBXW7)-mediated ubiquitination and proteasomal degradation of NR1D1.
(Microbial infection) Acts as a receptor for hepatitis C virus (HCV) in hepatocytes and facilitates its cell entry.
Phosphorylation at Thr-161 by CAK/CDK7 activates kinase activity. Phosphorylation at Thr-14 and Tyr-15 by PKMYT1 prevents nuclear translocation. Phosphorylation at Tyr-15 by WEE1 and WEE2 inhibits the protein kinase activity and acts as a negative regulator of entry into mitosis (G2 to M transition). Phosphorylation by PKMYT1 and WEE1 takes place during mitosis to keep CDK1-cyclin-B complexes inactive until the end of G2. By the end of G2, PKMYT1 and WEE1 are inactivated, but CDC25A and CDC25B are activated. Dephosphorylation by active CDC25A and CDC25B at Thr-14 and Tyr-15, leads to CDK1 activation at the G2-M transition. Phosphorylation at Tyr-15 by WEE2 during oogenesis is required to maintain meiotic arrest in oocytes during the germinal vesicle (GV) stage, a long period of quiescence at dictyate prophase I, leading to prevent meiotic reentry. Phosphorylation by WEE2 is also required for metaphase II exit during egg activation to ensure exit from meiosis in oocytes and promote pronuclear formation. Phosphorylated at Tyr-4 by PKR/EIF2AK2 upon genotoxic stress. This phosphorylation triggers CDK1 polyubiquitination and subsequent proteolysis, thus leading to G2 arrest. In response to UV irradiation, phosphorylation at Tyr-15 by PRKCD activates the G2/M DNA damage checkpoint.
Polyubiquitinated upon genotoxic stress.
Nucleus. Cytoplasm. Mitochondrion. Cytoplasm>Cytoskeleton>Microtubule organizing center>Centrosome. Cytoplasm>Cytoskeleton>Spindle.
Note: Cytoplasmic during the interphase. Colocalizes with SIRT2 on centrosome during prophase and on splindle fibers during metaphase of the mitotic cell cycle. Reversibly translocated from cytoplasm to nucleus when phosphorylated before G2-M transition when associated with cyclin-B1. Accumulates in mitochondria in G2-arrested cells upon DNA-damage.
Isoform 2 is found in breast cancer tissues.
Belongs to the protein kinase superfamily. CMGC Ser/Thr protein kinase family. CDC2/CDKX subfamily.
Serine/threonine-protein kinase involved in the control of the cell cycle; essential for meiosis, but dispensable for mitosis. Phosphorylates CTNNB1, USP37, p53/TP53, NPM1, CDK7, RB1, BRCA2, MYC, NPAT, EZH2. Triggers duplication of centrosomes and DNA. Acts at the G1-S transition to promote the E2F transcriptional program and the initiation of DNA synthesis, and modulates G2 progression; controls the timing of entry into mitosis/meiosis by controlling the subsequent activation of cyclin B/CDK1 by phosphorylation, and coordinates the activation of cyclin B/CDK1 at the centrosome and in the nucleus. Crucial role in orchestrating a fine balance between cellular proliferation, cell death, and DNA repair in human embryonic stem cells (hESCs). Activity of CDK2 is maximal during S phase and G2; activated by interaction with cyclin E during the early stages of DNA synthesis to permit G1-S transition, and subsequently activated by cyclin A2 (cyclin A1 in germ cells) during the late stages of DNA replication to drive the transition from S phase to mitosis, the G2 phase. EZH2 phosphorylation promotes H3K27me3 maintenance and epigenetic gene silencing. Phosphorylates CABLES1 (By similarity). Cyclin E/CDK2 prevents oxidative stress-mediated Ras-induced senescence by phosphorylating MYC. Involved in G1-S phase DNA damage checkpoint that prevents cells with damaged DNA from initiating mitosis; regulates homologous recombination-dependent repair by phosphorylating BRCA2, this phosphorylation is low in S phase when recombination is active, but increases as cells progress towards mitosis. In response to DNA damage, double-strand break repair by homologous recombination a reduction of CDK2-mediated BRCA2 phosphorylation. Phosphorylation of RB1 disturbs its interaction with E2F1. NPM1 phosphorylation by cyclin E/CDK2 promotes its dissociates from unduplicated centrosomes, thus initiating centrosome duplication. Cyclin E/CDK2-mediated phosphorylation of NPAT at G1-S transition and until prophase stimulates the NPAT-mediated activation of histone gene transcription during S phase. Required for vitamin D-mediated growth inhibition by being itself inactivated. Involved in the nitric oxide- (NO) mediated signaling in a nitrosylation/activation-dependent manner. USP37 is activated by phosphorylation and thus triggers G1-S transition. CTNNB1 phosphorylation regulates insulin internalization. Phosphorylates FOXP3 and negatively regulates its transcriptional activity and protein stability (By similarity). Phosphorylates CDK2AP2. Phosphorylates ERCC6 which is essential for its chromatin remodeling activity at DNA double-strand breaks.
Phosphorylated at Thr-160 by CDK7 in a CAK complex. Phosphorylation at Thr-160 promotes kinase activity, whereas phosphorylation at Tyr-15 by WEE1 reduces slightly kinase activity. Phosphorylated on Thr-14 and Tyr-15 during S and G2 phases before being dephosphorylated by CDC25A.
Nitrosylated after treatment with nitric oxide (DETA-NO).
Cytoplasm>Cytoskeleton>Microtubule organizing center>Centrosome. Nucleus>Cajal body. Cytoplasm. Endosome.
Note: Localized at the centrosomes in late G2 phase after separation of the centrosomes but before the start of prophase. Nuclear-cytoplasmic trafficking is mediated during the inhibition by 1,25-(OH)(2)D(3).
Belongs to the protein kinase superfamily. CMGC Ser/Thr protein kinase family. CDC2/CDKX subfamily.
Research Fields
· Cellular Processes > Cell growth and death > Cell cycle. (View pathway)
· Cellular Processes > Cell growth and death > Oocyte meiosis. (View pathway)
· Cellular Processes > Cell growth and death > p53 signaling pathway. (View pathway)
· Cellular Processes > Cell growth and death > Cellular senescence. (View pathway)
· Cellular Processes > Cellular community - eukaryotes > Gap junction. (View pathway)
· Environmental Information Processing > Signal transduction > FoxO signaling pathway. (View pathway)
· Environmental Information Processing > Signal transduction > PI3K-Akt signaling pathway. (View pathway)
· Human Diseases > Infectious diseases: Viral > Hepatitis B.
· Human Diseases > Infectious diseases: Viral > Measles.
· Human Diseases > Infectious diseases: Viral > Human papillomavirus infection.
· Human Diseases > Infectious diseases: Viral > Herpes simplex infection.
· Human Diseases > Infectious diseases: Viral > Epstein-Barr virus infection.
· Human Diseases > Cancers: Overview > Pathways in cancer. (View pathway)
· Human Diseases > Cancers: Overview > Viral carcinogenesis.
· Human Diseases > Cancers: Specific types > Prostate cancer. (View pathway)
· Human Diseases > Cancers: Specific types > Small cell lung cancer. (View pathway)
· Human Diseases > Cancers: Specific types > Gastric cancer. (View pathway)
· Organismal Systems > Endocrine system > Progesterone-mediated oocyte maturation.
References
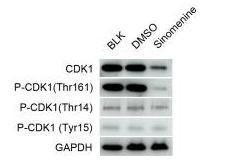
Application: WB Species: Human Sample: HeyA8 cells
Restrictive clause
Affinity Biosciences tests all products strictly. Citations are provided as a resource for additional applications that have not been validated by Affinity Biosciences. Please choose the appropriate format for each application and consult Materials and Methods sections for additional details about the use of any product in these publications.
For Research Use Only.
Not for use in diagnostic or therapeutic procedures. Not for resale. Not for distribution without written consent. Affinity Biosciences will not be held responsible for patent infringement or other violations that may occur with the use of our products. Affinity Biosciences, Affinity Biosciences Logo and all other trademarks are the property of Affinity Biosciences LTD.